6 7
CONTENTS
Quick Start Guide .........................................................................................8
Getting to Know Tala™ ............................................................................10
What’s Inside ................................................................................................. 11
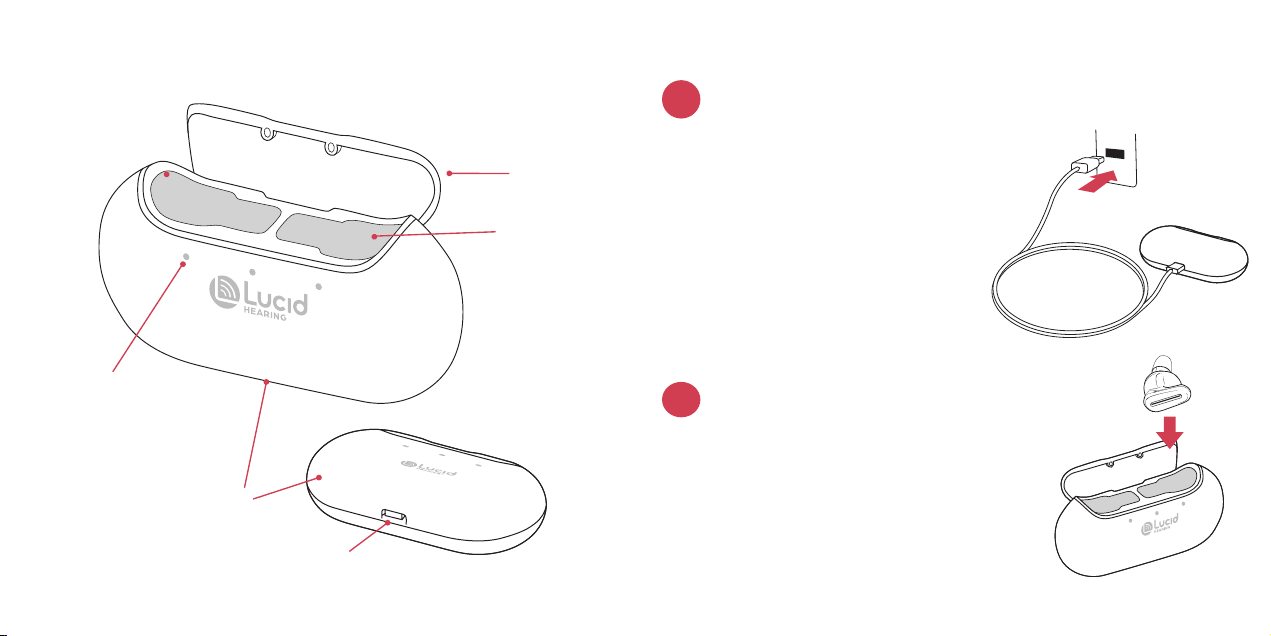
Getting to Know Tala™ Charging Case...........................................12
Charging Tala™ ............................................................................................13
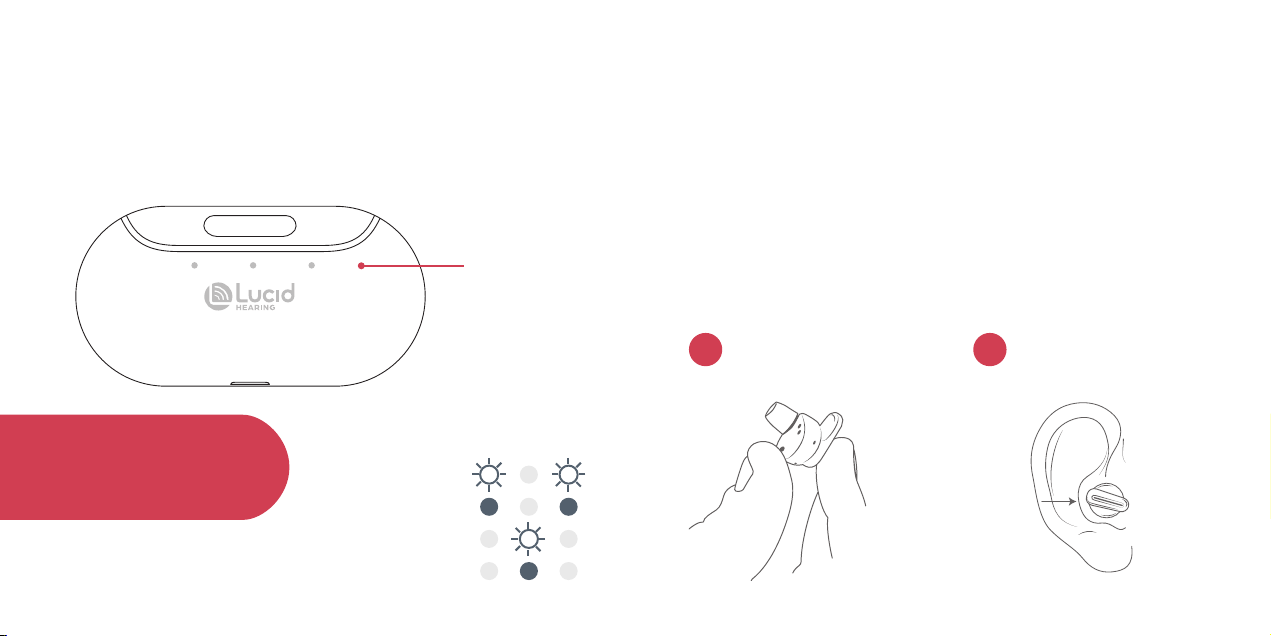
Charging Lights ...........................................................................................14
Wearing Your New Hearing Aids ........................................................15
Download the Lucid Hearing App .....................................................16
How to Adjust Volume and Programs .............................................17
Start-Up and Pairing ........................................................................ 18-19
Care and Cleaning Instructions ...................................................20-21
How to Clean Tala™ Hearing Aids .....................................................19
Replacing Wax Stop ........................................................................ 22-23
Choosing the Correct Ear Tip .............................................................. 24
Replacing the Ear Tips ............................................................................25
Troubleshooting ................................................................................. 26-27
Technical Data ............................................................................................28
Warranty ........................................................................................................29
Compliance ............................................................................................30-31
Battery Safety .................................................................................... 32-33
in situations that make you tired from listeningfor
example, noisy environments.
Note: Tell FDA about injuries, malfunctions,
or other adverse events.
To report a problem involving your hearing aid,
you should submit information to FDA as soon
as possible after the problem. FDA calls them
“adverse events,” and they might include: skin
irritation in your ear, injury from the device (like
cuts or scratches, or burns from an overheated
battery), pieces of the device getting stuck in
your ear, suddenly worsening hearing loss
from using the device, etc.
Instructions for reporting are available at
https//www. fda.gov/Safety/MedWatch,
or call 1–800-FDA-1088. You can also download
a form to mail to FDA.