User Guide ergo basic
Table of Contents
1 Safety Notes ......................................................................... 4
1.1 Responsibility of the User ................................................... 4
1.2 Organisational Measures ..................................................... 4
1.3 Indications for Use ............................................................... 4
1.4 Contra-indication ................................................................. 4
1.5 Safety-conscious Operation ................................................. 5
1.6 Safe Use with Electronics .................................................... 5
1.7 Operation with other Devices .............................................. 5
1.8 Maintenance ........................................................................ 5
1.9 Terms of Warranty ............................................................... 6
1.10 Symbols and Pictograms ..................................................... 6
1.10.1 Symbols Used in this Document ................................................ 6
1.10.2 Symbols Used on the Device........................................................ 7
2 Introduction ......................................................................... 8
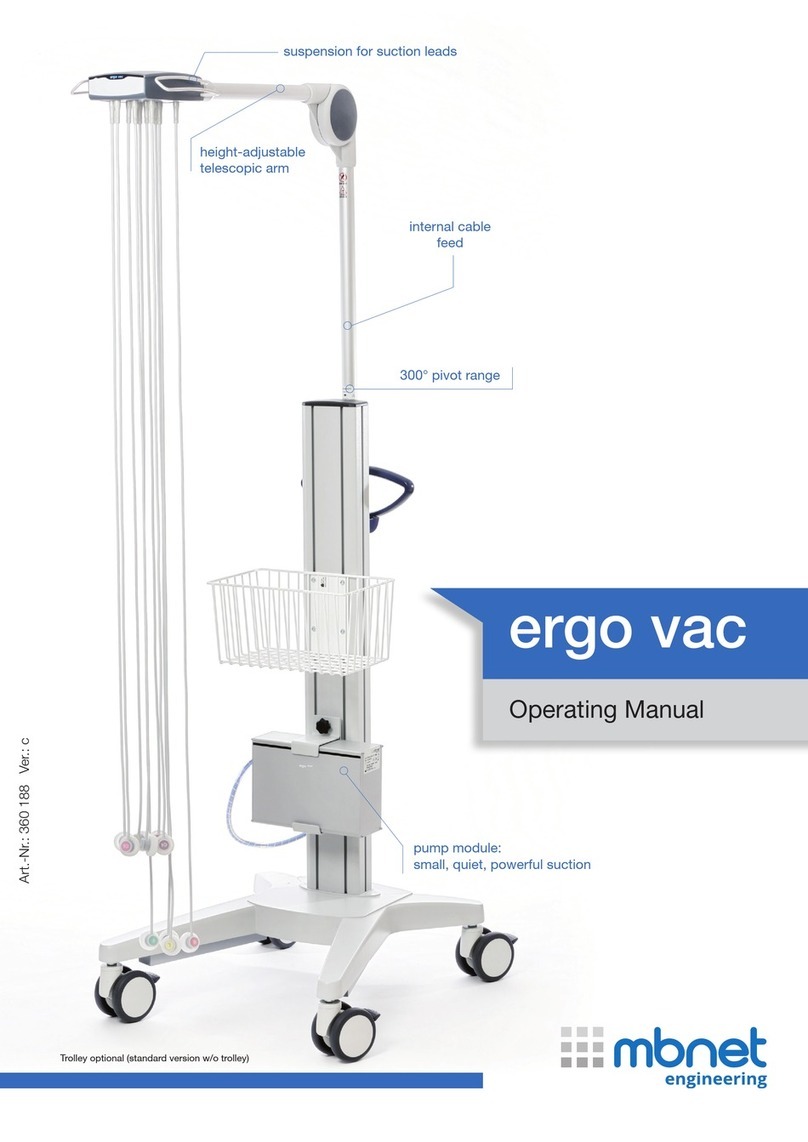
2.1 Elements of the Suction Device........................................... 8
2.1.1 Overview .......................................................................................... 8
2.1.2 Scope of Delivery ............................................................................ 8
2.2 Patient module..................................................................... 9
2.3 Joint...................................................................................... 9
2.4 Telescopic arm closure ...................................................... 10
2.5 Leads................................................................................... 10
2.6 Fixation of cable arm.......................................................... 10
2.7 Serial Number .................................................................... 11
2.8 Supplied accessories for ergo basic .................................. 11
3 Operation ........................................................................... 11
3.1 Getting Started and Location ............................................ 11
4 ECG Recording ................................................................... 12
4.1 Placement of Electrodes ................................................... 12
4.2 Possible Sources of Errors with the ECG Recording ......... 13
4.2.1 Preparation ................................................................................... 13
4.2.2 Application of Electrodes ........................................................... 13
4.2.3 Before the Recording ................................................................... 13
4.2.4 During the Recording ................................................................... 13
4.2.5 Removal of Electrodes from the Skin........................................ 13
4.3 Electrode Identification and Colour Code ........................ 14
4.4 Resting ECG with 10-lead Patient Cable
Electrode Placement for Standard Leads .................. 14 – 15
4.5 Right Precordial (C4r).................................................. 15 – 16
5 Application .......................................................................... 16
5.1 Operating Conditions ........................................................ 16
5.2 Starting a Recording .......................................................... 17
6 Maintenance and Care ........................................................ 17
6.1 Visual Inspection........................................................................... 17
6.2 Cleaning the Housing and Cables ............................................ 18
6.2.1 Cleaning the ECG-leads .............................................................. 19
6.2.2 Cleaning Connection Cables...................................................... 19
6.2.3 Admissible Detergents................................................................. 19
6.2.4 Non-admissible Detergents ........................................................19
6.3 Disinfection ........................................................................ 20
6.3.1 Admissible Disinfectants ............................................................ 20
6.3.2 Non-admissible Disinfectants .................................................... 20
6.4 Inspection Report .............................................................. 21
6.5 Accessories and Consumables .......................................... 22
6.6 Replacement of ECG Leads ............................................... 22
7 Troubleshooting................................................................. 22
7.1 Possible Errors ................................................................... 22
7.2 Preventing Electromagnetic Interference ........................ 23
7.3 Warranty ............................................................................. 24
7.4 Accessories and Disposables ............................................. 24
8 Technical Data ................................................................... 25
8.1 Device ................................................................................. 25
8.2 System Cable ..................................................................... 25
8.3 Cable Arm ........................................................................... 25
8.4 Safety Standards ............................................................... 25