Table of Contents
DISCLAIMER.......................................................................................................................................................................................................................................1
TRADEMARKS ...................................................................................................................................................................................................................................1
1 PREFACE ......................................................................................................................................................................................................................................1
1.1 Intended Purpose........................................................................................................................................................................................................1
1.2 Target Patient Group and Intended Users.......................................................................................................................................................2
1.3 Clinical Benets ............................................................................................................................................................................................................2
1.4 Explanation of Safety Warnings ...........................................................................................................................................................................2
1.5 Retention Instructions...............................................................................................................................................................................................2
1.6 Obtaining Documentation and Information..................................................................................................................................................2
1.6.1 Ordering Documentation...........................................................................................................................................................................2
1.6.2 Other languages.............................................................................................................................................................................................2
1.6.3 Documentation Feedback..........................................................................................................................................................................2
1.6.4 Support and service......................................................................................................................................................................................2
1.6.5 Name and address of the manufacturer ............................................................................................................................................3
2 DESCRIPTION OF THE PRODUCT .....................................................................................................................................................................................3
2.1 Intended Use and Reasonably Foreseeable Misuse....................................................................................................................................3
2.2 Sterilization State and Method.............................................................................................................................................................................3
2.3 Summary of Safety and Clinical Performance...............................................................................................................................................3
2.4 Technical Characteristics..........................................................................................................................................................................................4
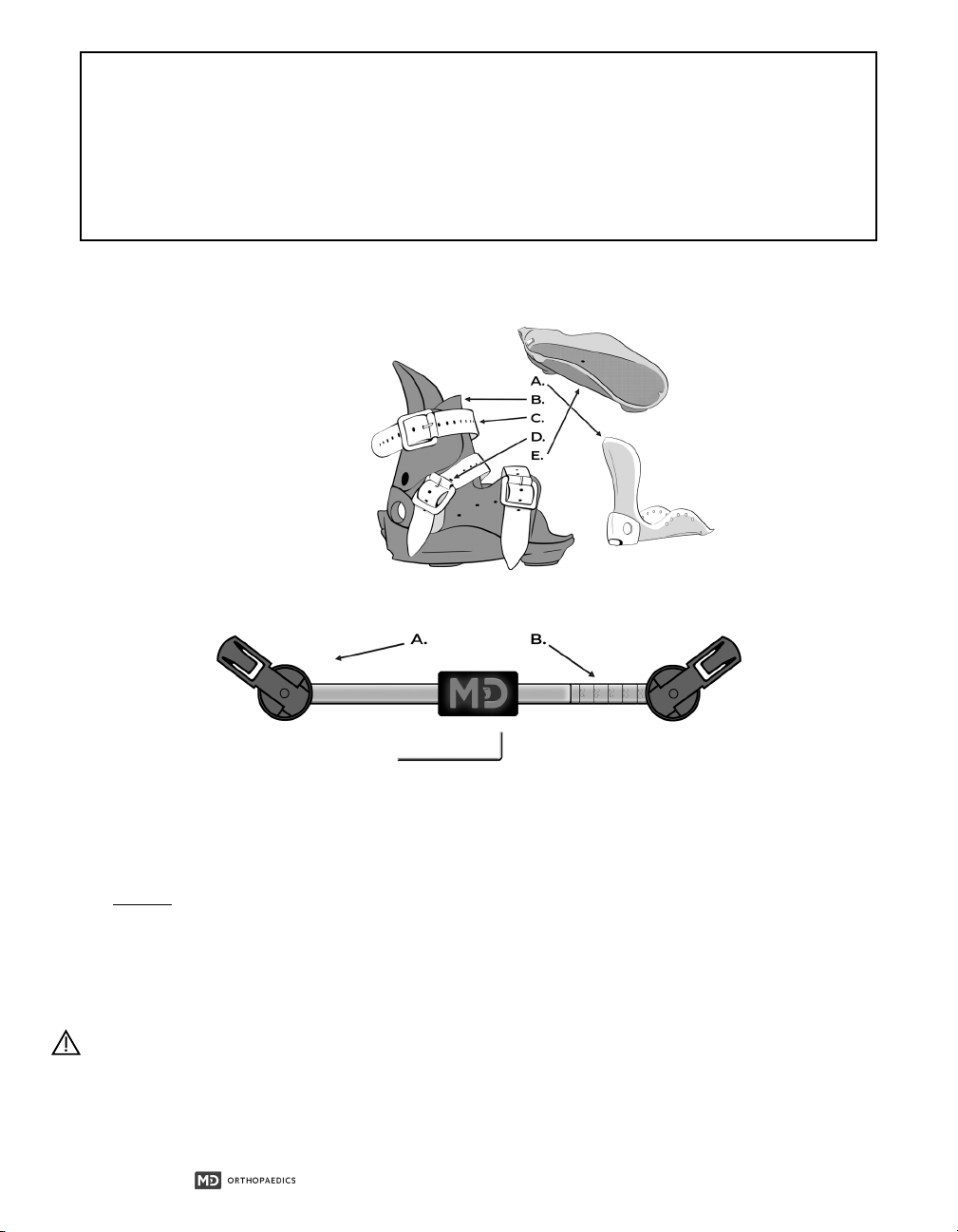
2.4.1 Mitchell Ponseti® Ankle Foot Orthotic.................................................................................................................................................5
2.4.2 Ponseti® Abduction Bar...............................................................................................................................................................................5
3 SAFETY INSTRUCTIONS .......................................................................................................................................................................................................5
3.1 How to Use the Product Safely..............................................................................................................................................................................6
3.1.1 Technical life span and Warranty............................................................................................................................................................6
3.1.2 Safety information related to the intended use and reasonably foreseeable misuse.................................................6
3.1.3 Product limitations and restrictions and contraindications.....................................................................................................6
3.1.4 Safety information when using the device in combination with other devices..............................................................6
3.1.5 Safety information regarding the use .................................................................................................................................................6
3.1.6 Safe Disposal....................................................................................................................................................................................................6
3.2 Potential Health Consequences............................................................................................................................................................................6
4 INSTRUCTIONS FOR USE.....................................................................................................................................................................................................7
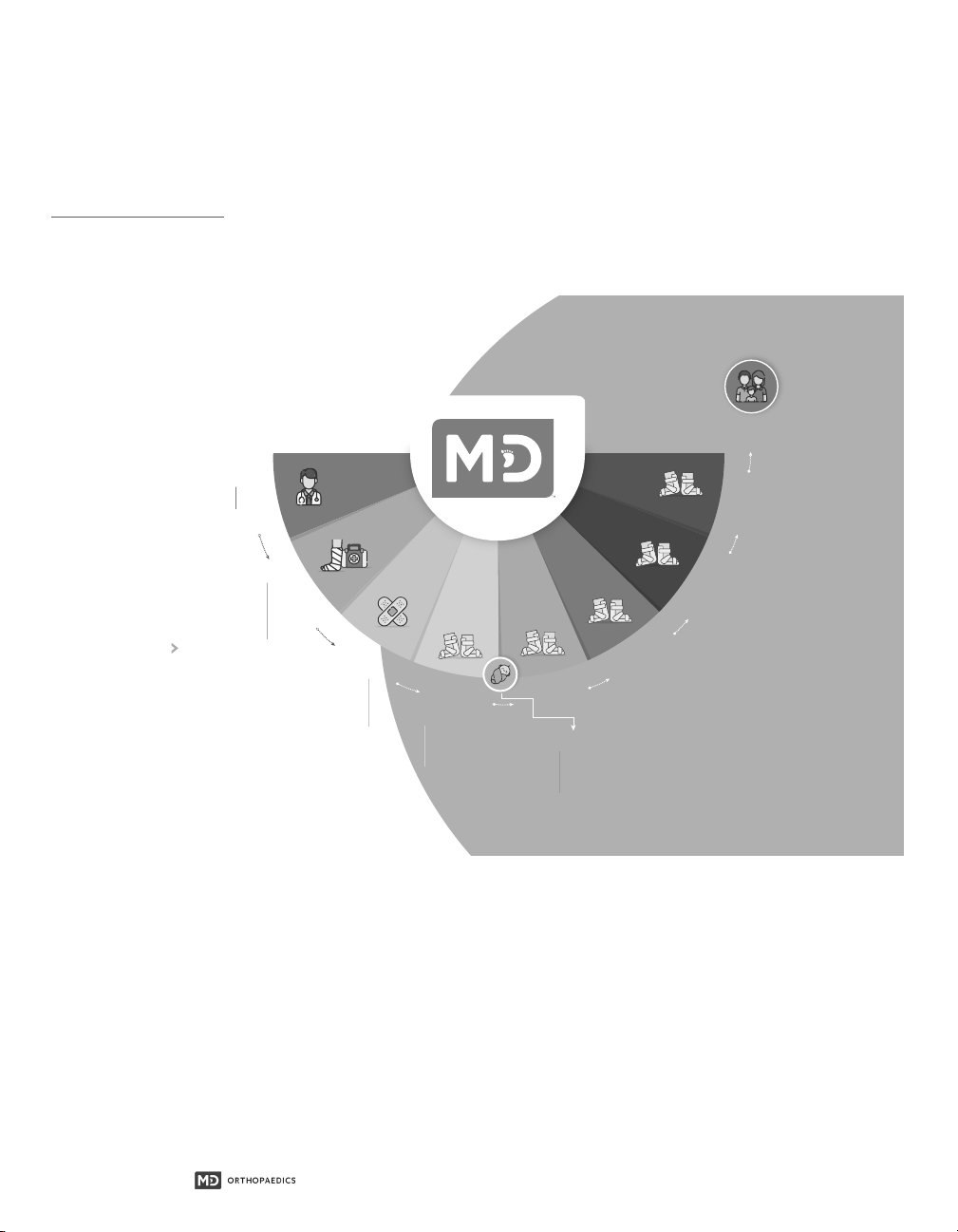
4.1 Clubfoot Treatment Overview...............................................................................................................................................................................7
4.2 Mitchell Ponseti® AFO................................................................................................................................................................................................8
4.3 Ponseti® Abduction Bar ............................................................................................................................................................................................9
5 PREPARATION..........................................................................................................................................................................................................................9
5.1 How to Transport and Store the Product..........................................................................................................................................................9
6 MAINTENANCE.........................................................................................................................................................................................................................9
6.1 Reusing the Device .....................................................................................................................................................................................................9
6.1.1 Cleaning the device.......................................................................................................................................................................................9
6.2 How to Inspect the Product ....................................................................................................................................................................................9
7 TROUBLESHOOTING .......................................................................................................................................................................................................... 10
7.1 How to Identify and Solve Problems............................................................................................................................................................... 10
7.1.1 Troubleshooting by non-skilled persons......................................................................................................................................... 10
7.2 Frequently Asked Questions............................................................................................................................................................................... 10
8 GLOSSARY ............................................................................................................................................................................................................................... 11
9 ICON LEGEND......................................................................................................................................................................................................................... 12