WARNING (referred to possible damage to property)
The purpose of this manual is to ensure that operators are aware of the safety requirements, of the
installation procedures and of the instructions for correct use and maintenance of the apparatus.
The user is not authorised to tamper with the equipment under any circumstances.
If any problems are encountered, please contact a Mectron Service Centre.
Any attempts on the part of the user or any unauthorised personnel to tamper with or alter the
apparatus will invalidate the warranty and release the Manufacturers from any liability in respect of
any harm or damage to persons or property.
The information and illustrations contained in this manual are up-dated to the date of publication
indicated on the last page.
MECTRON are committed to continuous up-dating of their products, which may entail changes to
components of the equipment. If there are any discrepancies between the descriptions contained
in this manual and your equipment, please contact your dealer or the MECTRON After-Sale service
for explanations.
Using this manual for purposes other than those relating to the installation, use and maintenance of
the equipment is strictly prohibited.
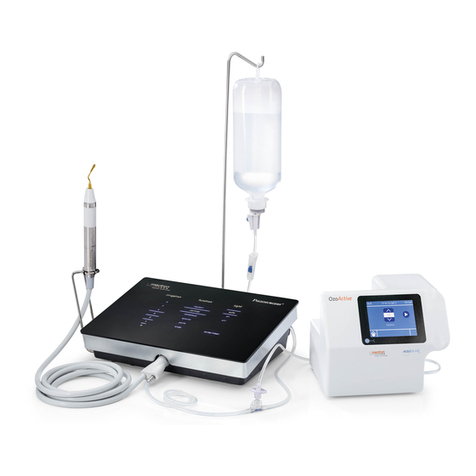
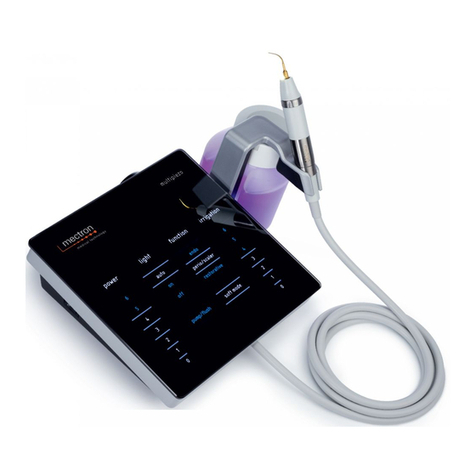
00.2 Description of the Device
Thanks to its controlled three-dimensional ultrasound oscillations, the original Piezosurgery tech-
nique rings in a new age for osteotomy and osteoplasty in Implantology, Periodontology, Endodon-
tics and Orthodontic Surgery. Its main features are:
- Micrometric cutting: Maximum surgical precision and intra-operative sensibility;
- Selective cutting: Maximum safety for the soft tissues;
- Cavitation effect: Maximum intra-operative visibility (bloodless eld).
The equipment has an automatic tuning circuit that offsets wear of the inserts, thus ensuring work
in constant conditions of maximum efciency.
Summary
00.0 Introduction........................................................................................................................ .....3
00.1 Foreword .................................................................................................................... .....3
00.2 Description of the Device............................................................................................ .....3
00.3 Intended Use .............................................................................................................. .....4
00.4 Safety requirements .................................................................................................. .....4
01.0 Identication data.............................................................................................................. .....6
01.1 Identication data ...................................................................................................... .....6
01.2 Data plate of the device.............................................................................................. .....6
01.3 Data plate of the scaler handpiece ............................................................................. .....6
02.0 Testing ................................................................................................................................ .....7
02.1 Testing of the equipment ............................................................................................ .....7
03.0 Delivery............................................................................................................................... .....7
03.1 Delivery of the apparatus............................................................................................ .....7
03.2 List of material included in the supply......................................................................... .....8
04.0 Installation.......................................................................................................................... ...10
04.1 Safety requirements during Installation ...................................................................... ...10
04.2 Initial installation ......................................................................................................... ...10
04.3 Connecting the Accessories ....................................................................................... ...11
05.0 Use...................................................................................................................................... ...13
05.1 Controls ...................................................................................................................... ...13
05.2 Switching the device ON and OFF ............................................................................ ...13
05.3 Description of the display and functions .................................................................... ...14
05.4 Safety requirements during use ................................................................................. ...16
05.5 Protection systems and alarms ................................................................................. ...17
05.6 Instructions for use .................................................................................................... ...18
05.7 Permissible settings on the basis of the type of insert................................................ ...19
05.8 Rules for keeping the device in proper working order ................................................ ...19
06.0 Cleaning and sterilisation................................................................................................. ...20
06.1 CLEAN function - Cleaning of the liquid circuit........................................................... ...20
06.2 Cleaning and disinfecting the casing of the device..................................................... ...20
06.3 Sterilisation procedure ............................................................................................... ...21
06.4 Cleaning and autoclave sterilisation of the handpiece ............................................... ...21
06.5 Cleaning and autoclave sterilisation of the inserts ..................................................... ...22
06.6 Cleaning and autoclave sterilisation of the wrench for tightening the inserts ............. ...23
06.7 Autoclave sterilising of the tube of the peristaltic pump.............................................. ...23
06.8 Autoclave sterilising of the tting between the cord and the tube of the peristaltic pump . ...24
07.0 Regular maintenance ....................................................................................................... ...24
07.1 Shelf Storage.............................................................................................................. ...24
07.2 Power-supply cable ................................................................................................... ...24
08.0 Replacement of the fuses................................................................................................. ...25
09.0 Disposal procedures and precautions ............................................................................ ...25
10.0 The inserts ......................................................................................................................... ...26
11.0 Symbols.............................................................................................................................. ...26
12.0 Troubleshooting ............................................................................................................... ...27
13.0 Technical data.................................................................................................................... ...30
13.1 Electromagnetic compatibility EN 60601-1-2............................................................... ...31
14.0 Guarantee........................................................................................................................... ...35