4
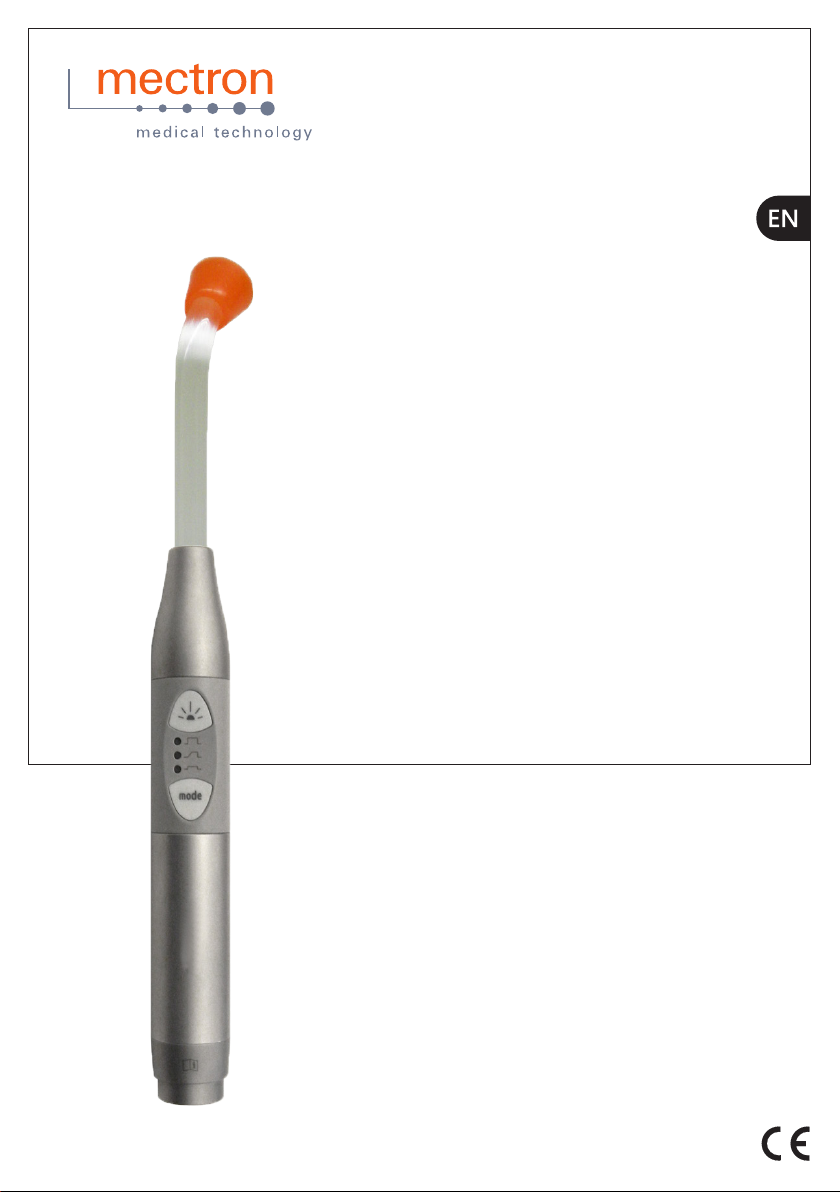
STARLIGHT S +
1.4 Safety Requirements
CAUTION: No alterations to this device are permitted.
CAUTION: The electrical system of the premises in which the device is installed and used
must comply with the rules in force and the relevant requirements of electrical safety.
WARNING: Qualiedandspecialisedpersonnel.
training activities are foreseen for the use of the device. The use of the device does not cause
WARNING: Intended use.
Use the equipment solely for the purpose for which it is intended (see
Chapter 1.1 on page
2
). Failure to comply with this requirement could lead to serious harm to the patient and/or
to the operator and/or damage to/failure of the equipment.
WARNING: Contraindications.
Do not use the device on patients with Pacemakers or other implantable electronic devices.
This regulation also applies to the operator.
WARNING: Pointthebeamoflightdirectlyatthematerialtobepolymerised.
Do not point the beam of light on the gums or other soft tissues (if necessary these parts
cavity to be clinically treated..
WARNING: Neverpointthebeamoflightontheeyes.
WARNING: Contraindications.
Do not use this equipment for patients who have a case history of positive reaction to
with photosensitising drugs. In all cases of possible risk consult a specialised physician.
WARNING: Contraindications.
Adopt strict safety measures for patients who have undergone cataract surgery and who are
WARNING: Contraindications.
Patients who have a case history of diseases of the retina should consult their optician
.
CAUTION: PhotobiologicalsafetyofthecuringlightsandlampsystemsIEC62471.
concerning a retinal risk from blue light or thermal retinal risk.
The following CAUTION indications are applied to the device package.