PRECAUTIONS
1. Only use the device for its intended purpose as described in this instruc-
tion manual, therefore as a compressor nebulizer system for aerosolthe-
rapy, following the indications of your doctor. Any other form of use constitutes
an improper use and is therefore dangerous. The manufacturer cannot be held liable for
any damage caused by improper, incorrect or unreasonable use, or if the unit is connected
to electrical installations which do not conform with safety regulations.
2. Keep this manual for future reference.
3. Do not operate the unit in presence of any anesthetic, inflammable mixtures, oxygen or
nitrogen monoxide.
4. The correct device functioning can be affected by electromagnetic interference which
exceeds the limits indicated by the European standards in force. In case this device interfe-
res with other electrical apparatus, remove it and plug it to a different socket.
5. In case of failure and/or malfunction, read the ”POSSIBLE PROBLEMS AND HOW TO
SOLVE THEM” section. Do not handle nor open the compressor housing.
6.
Any repairs must be carried out by an authorized dealer using only original spare parts.
Safety of the device can be compromised in case the above mentioned indications are not met.
7. When using any electrical appliance certain important safety measures must always be
observed, including the following:
- use only original accessories and components;
- never submerge the unit in water;
- the device is not protected against water penetration;
- never touch the unit when your hands are wet or moist;
- do not leave the unit outdoor;
- the unit, when operating, must be placed on a stable and horizontal surface;
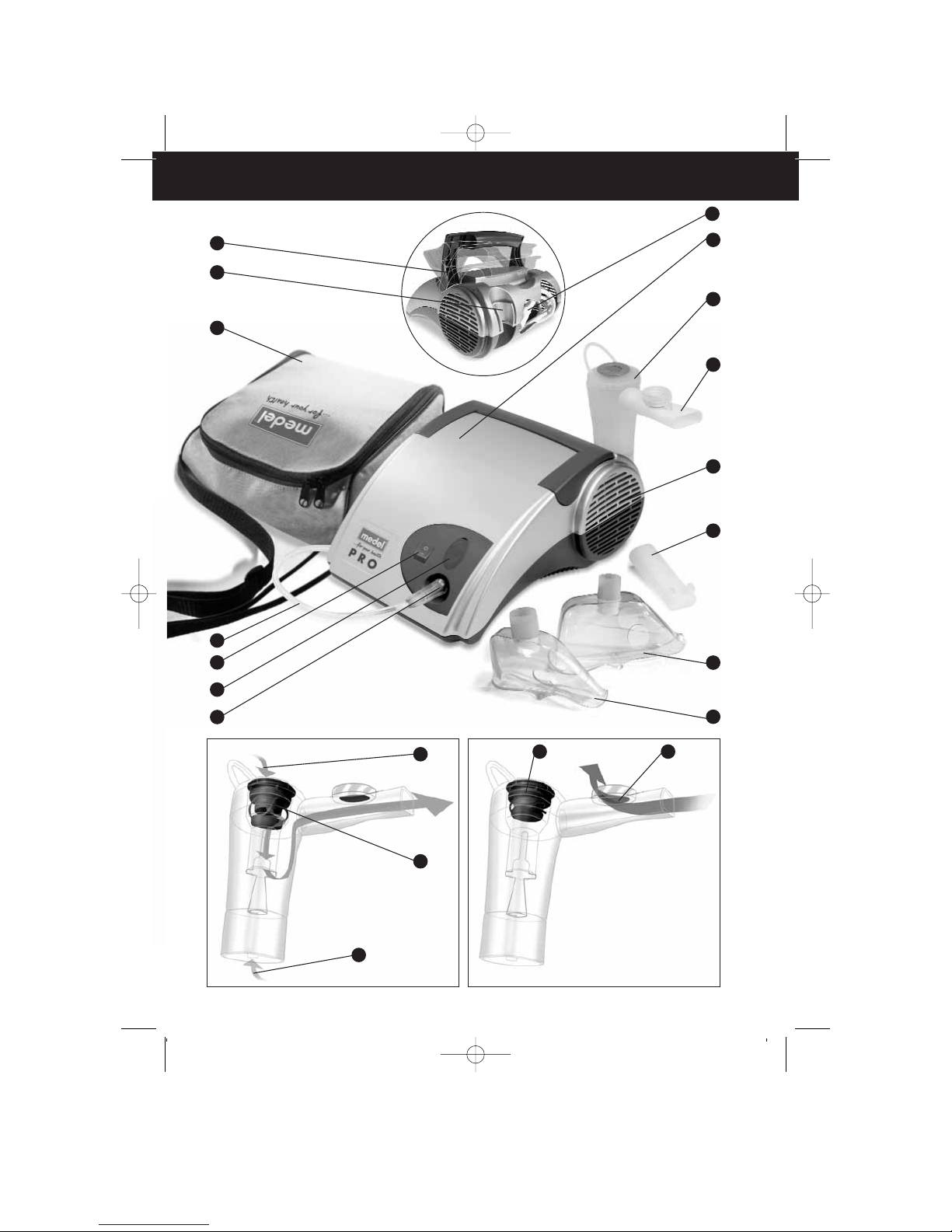
- make sure the air vent openings are not obstructed (pic. A/7);
- do not allow unsupervised children or infirm persons to use the unit;
- do not disconnect the unit by simply pulling out the plug from the wall socket.
8. Make sure that the electrical rating shown on the rating plate on the bottom of the unit cor-
responds to your main voltage and frequency before plugging in the device.
9. If the power plug provided with the device does not fit your wall socket, have the plug chan-
ged by an electrical appliance dealer. Do not use any adapter, simple or multiple, and/or
extension cable. In case their use is necessary, make sure they are in compliance with safety
standards, paying attention they do not exceed the maximum load.
10. Do not leave the unit plugged when not in use: unplug the device from the wall socket when
not operating.
11.
Follow the manufacturer’s instructions for installing the device. The manufacturer cannot be held
responsible for any damage caused to persons, animals or things by incorrect installation.
12. The power supply cord cannot be replaced by the user. In case the power supply cord beco-
mes damaged, address to the technical servicing authorized by the manufacturer for repla-
cement.
13. The power supply cord should always be fully unwound in order to prevent dangerous
12
UK