Meditrac Cervico 2000 User manual

Cervico 2000
User Manual
1
© All rights reserved to Meditrac Ltd.

TABLE OF CONTENTS:
2
3Introduction1.
5Parts of the Cervico 20002.
7Instruction for Use of the Cervico 20003.
11Care and Maintenance of your Cervico 20004.
12Contact Information5.

1. INTRODUCTION
Congratulations on purchasing the latest concept in cervical spine treatment –the
Cervico 2000. Offering safe, simple, compact, and effective treatment of cervical
spine disorders, the Cervico 2000 brings to both the patient and physician a means
of powerful, dynamic traction with simultaneous, three-dimensional action
without the need for traction beds or restrictive braces. Thus, the Cervico 2000
allows the patient maximum mobility with minimal restrictions or discomfort
during treatment. Designed with the patient in mind, the Cervico 2000 facilitates
the treatment of the patient by the health service professional while also allowing
chronic sufferers to administer self-treatment under qualified medical supervision.
We wish you good luck and good health with your Cervico 2000.
FOR YOUR OWN HEALTH AND SAFETY
Please read the following and familiarize yourself with all of the instructions
before using your Cervico 2000.
Indications for use of the Cervico 2000:
•Neck pain as a result of discogenic disorders, osteoarthritis, or degenerative
changes in the cervical spine
•Whiplash injuries, usually as a result of traffic accident (injuries which do not
involve breakage or dislocation of the bones)
•Torticollis as a result of rheumatic or muscular pain or spastic torticollis with
a neurological basis
•Job-related neck pain (i.e. clerks, secretaries), prolonged computer use,
sewing, etc.
•Sports injuries (without breakage or dislocation)
3

Contra-indications for use of the Cervico 2000:
•Do not allow contact between the device and open wounds.
•Irritation of the skin on the jaw, neck, or shoulders
•Tumors or protrudences on the mandibula, cervical spine, or claviculae
•Breakage of the mandibula, cervical spine vertebrae, or claviculae, and
following recent surgery
•Infections of the mandibula, cervical spine vertebrae, or teeth
•Temporo-mandibula joint disorders, including pains, infections, or
subluxation
•Vascular disorders of the neck, partial clogging, or any kind of fusion, and
following recent surgery
•Following dental surgery or implants (false teeth should be taken out).
•Neoplasms.
Adverse Reactions:
Although extremely uncommon, if any of the reactions listed below are
experienced, immediately discontinue use of the Cervico 2000 and contact the
administering medical professional.
•Dizziness
•Breathing difficulties
•Headaches
•Abnormal fatigue
•Aggravation of symptoms
4
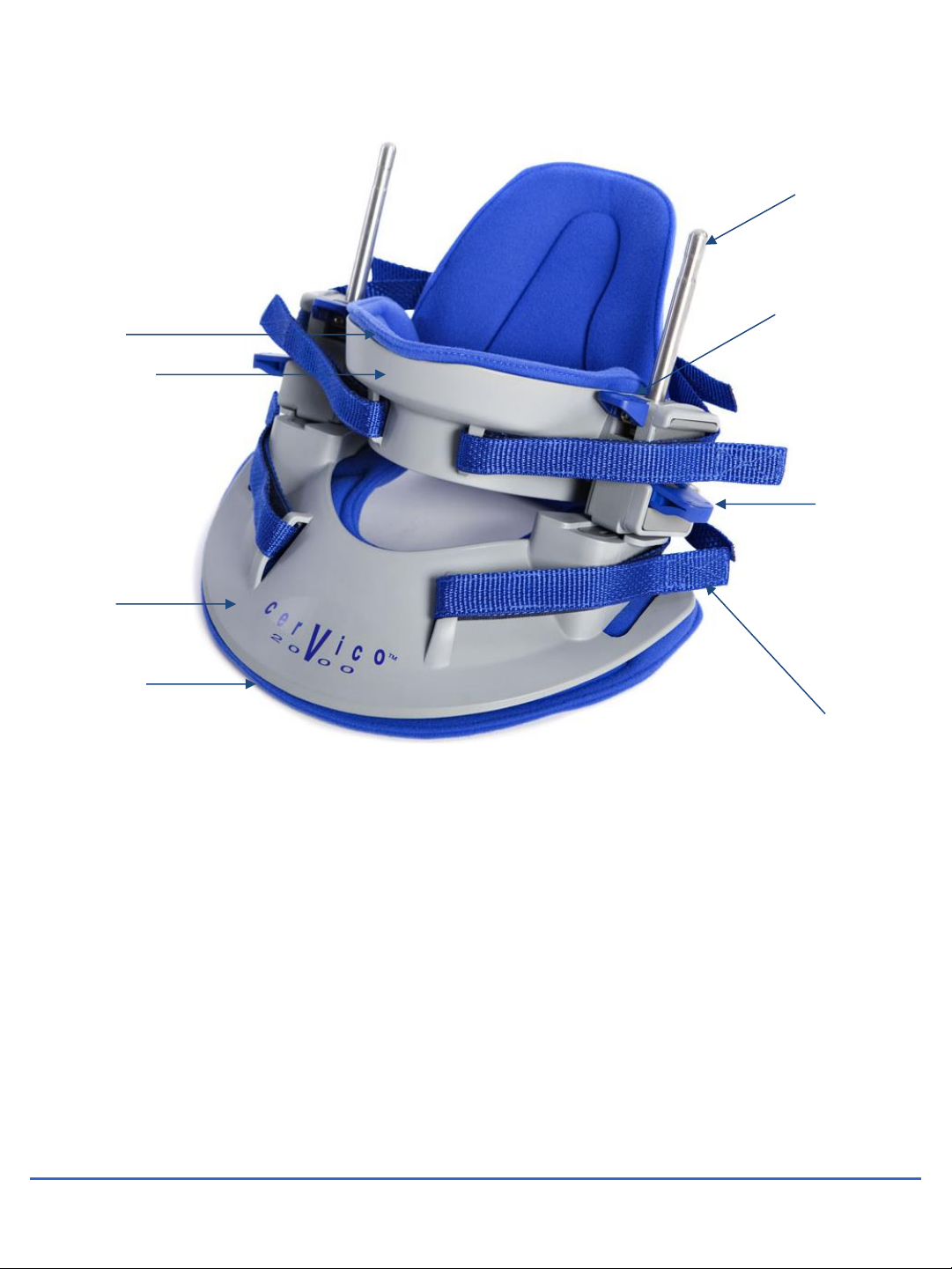
2. PARTS OF THE CERVICO 2000
5
1
2
3
4
5
6
7
8
1. Collar
2. Pad for collar
3. Chin support
4. Pad for chin support
5. Release handle
6. Traction lever
7. Straps
8. Traction rod

9. Brace
10. Pad for brace
11. Location for horizontal pressure screw
12. Horizontal pressure pad
13. Horizontal screw
9
10
12
13
11
6

3. INSTRUCTION FOR USE OF THE CERVICO 2000
Step 1: Remove all jewelry such as earrings, necklaces, and hair
accessories. Wash and dry the jaw and neck areas which will be
in contact with the Cervico 2000. The patient should wear a
comfortable shirt with no zippers, buttons, or protruding
decorations which may cause discomfort during treatment.
Step 2: Make sure that the jack mechanism is at the lowest
position and fits snugly into the traction rod housing of the
collar. This is the “Zero Position“.
Step 3: To fit the horizontal pressure screw, remove the pad
from the brace, then fit the horizontal screw through the upper
or lower location, as appropriate, so that the pressure pad is on
the inside and the screw thread is outside the device (Fig. 1).
Refit the pad and fit the screw head.
Step 4: While holding the device so that the patient can read
the words "Cervico 2000", make sure that the upper strap and
the lower strap on one side are open and that the straps on the
other side are attached by folding them back and securing them
against the Velcro fastening strips on the surfaces of the straps
(Fig. 2).
7
Fig. 1
Fig. 2

Step 5: While the device is still in the “Zero Position",
place the collar on the collarbones and shoulders so that
the front of the jaw rests gently on the chin support (Fig.
3). The collar has two channels through which the straps
should be threaded, folded back, and secured by the
Velcro fastenings located on the surfaces of the straps.
The collar and chin support should fit snugly, without pain
or discomfort. During self-treatment, it is recommended
that the patient fit the device in front of a mirror.
Step 6: With the device resting comfortably, it may be
necessary to adjust the placement of the collar, the brace,
or the tightness of the straps in order to ensure the
patient's comfort (Fig. 4). For larger or smaller patients, it
is possible to move the traction rods forward or backward
in the traction rod housing for a secure, comfortable fit.
Only after the device is properly positioned is it possible to
apply traction as necessary.
Step 7: When the patient is ready, begin applying traction
by gently lifting both traction levers simultaneously (Fig.
5). In cases of self-treatment it is recommended to use
the index and middle fingers to grasp the top of the
traction housing and then to apply firm pressure with the
thumb to lift the traction levers. Repeat until the skin of
the neck is taut and there is resistance to the traction of
the device. The patient should feel gentle traction, but
little or no discomfort. Always apply traction slowly, so as
to prevent discomfort.
8
Fig. 4
Fig. 5
Fig. 3

Step 8: When the desired amount of decompression has
been achieved, additional horizontal pressure can be
applied to restore the lordosis. Apply the required
horizontal pressure by turning the screw head gently in a
clockwise direction until the patient feels gentle pressure,
but little or no discomfort (Fig. 6).Always apply pressure
slowly, so as to prevent discomfort.
Step 9: It is possible to apply asymmetrical traction when
needed (such as to the left or to the right) by jacking one
side more than the other (Fig. 7 and Fig. 8). Again,
traction should be applied slowly to avoid discomfort.
Step 10:Traction should be performed for approximately
15-20 minutes per day. Treatment of acute cases with the
Cervico 2000 is recommended daily for approximately one
week, but not more than ten days. In chronic or severe
cases, it is recommended to undergo treatment twice daily
for approximately two weeks, but only under qualified
medical supervision. It is suggested that the patient walks
or participates in active exercises during the treatment
session (i.e. on a treadmill or using elastic bands).
9
Fig. 6
Fig. 7
Fig. 8

Step 11:Upon finishing the treatment session, release
the traction by pressing down on the release handles
located on the jack mechanisms on both sides of the
device (Fig. 9). Release traction slowly and
simultaneously on both sides in order to avoid causing
discomfort to the patient or damage to the device.
Step 12:Once the device has been returned to the
“Zero Position", release the upper and lower straps
and remove it while the patient is sitting. It is
recommended that the straps be released on one side
of the device only so that the brace does not
disconnect from the device and fall of the patient. The
device is now ready for the next treatment.
10
Fig. 9

4. CARE AND MAINTENANCE OF YOUR CERVICO 2000
While the Cervico 2000 applies powerful treatment forces to the cervical spine, it
is a precision device that needs proper care. Make sure to follow these simple
steps:
•Be sure to handle the device gently.
•Keep the device properly stored in the “Zero Position” in its original carrying
bag when not in use.
•Avoid exposing the device to water, excess humidity, or direct sunlight for
extended periods of time.
•Wash and dry the neck area of the patient before use.
•Before treatment, place a layer of tissue or sanitary paper on the device so
that there is minimal contact between the patient's body and the pads which
may become excessively soiled.
•Clean the device by wiping the plastic and polyurethane with a cloth
dampened with alcohol.
•Wipe the foam and polyurethane pads with alcohol after each use whether
the unit is being used for one patient, two patients, or twenty patients.
•In a clinic environment, it is recommended that spare sets of pads be kept on
hand for convenience and comfort of the patient. You can order them from
your Cervico 2000 representative or directly from Meditrac.
11

5. CONTACT INFORMATION
If you have any problems or questions regarding your Cervico 2000, please contact
us at:
Meditrac Ltd. Phone: +972-3-5467828
53 Pinkas St. Fax: +972-3-5467619
Tel Aviv 6226117 Toll-free: 1-866-732-0170
Israel E-mail: info@meditrac.co.il
Meditrac's products carry the CE mark. FDA and ISO:13485
and are registered with the United States Food and Drug Administration.
United States Federal Law permits the sale of these devices (within the US) by a
doctor’s prescription only
Model No. : CERV6
Version No.: 2019
12
Meditrac's authorized representative:
MDI Europa Gmbh
Langenhagener Str. 71
D-30855 Hannover-Langenhagen
Germany
Table of contents
Other Meditrac Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Drive DeVilbiss Healthcare
Drive DeVilbiss Healthcare 1275/PS/HI/EX Instructions for use
NHS
NHS Cough Assist manual

swissflex
swissflex Uni 20_45 operating instructions

Mindfield
Mindfield MindLights Instructions for installation and use

Pressalit Care
Pressalit Care R4820 Mounting instruction

Schiller
Schiller Cardiovit AT-10 Service handbook