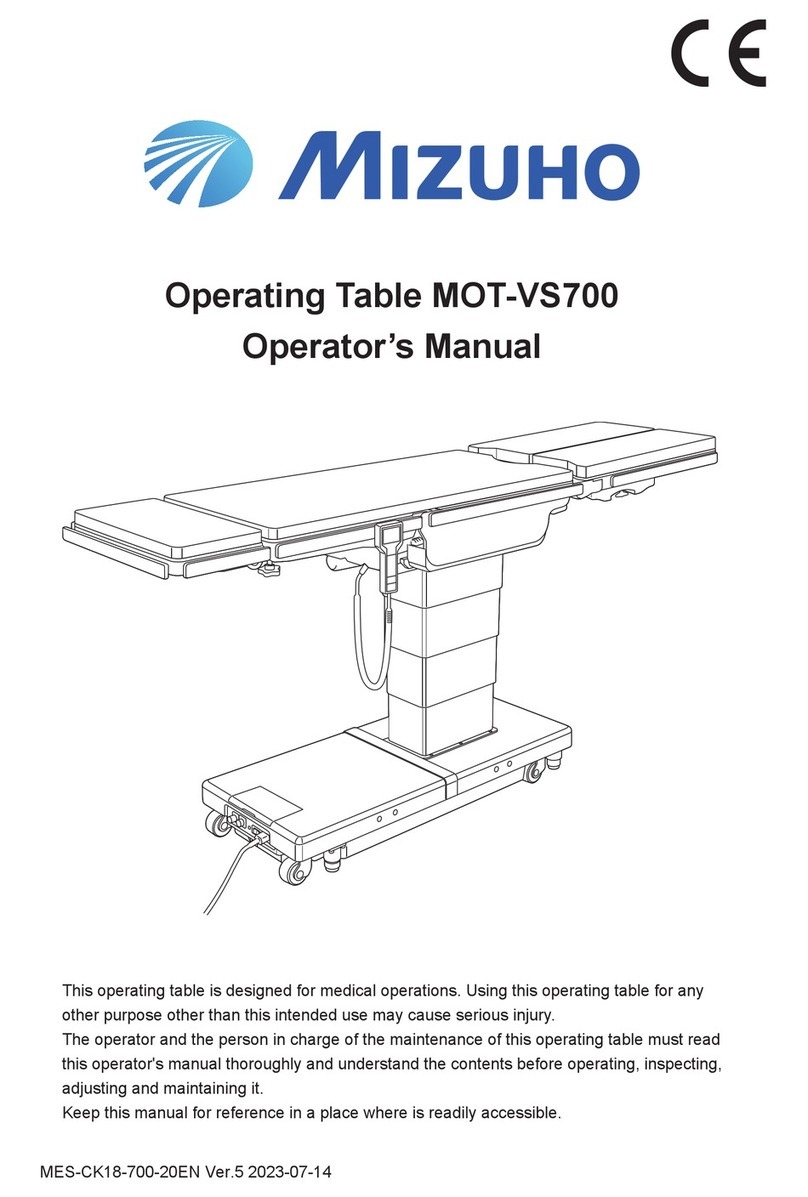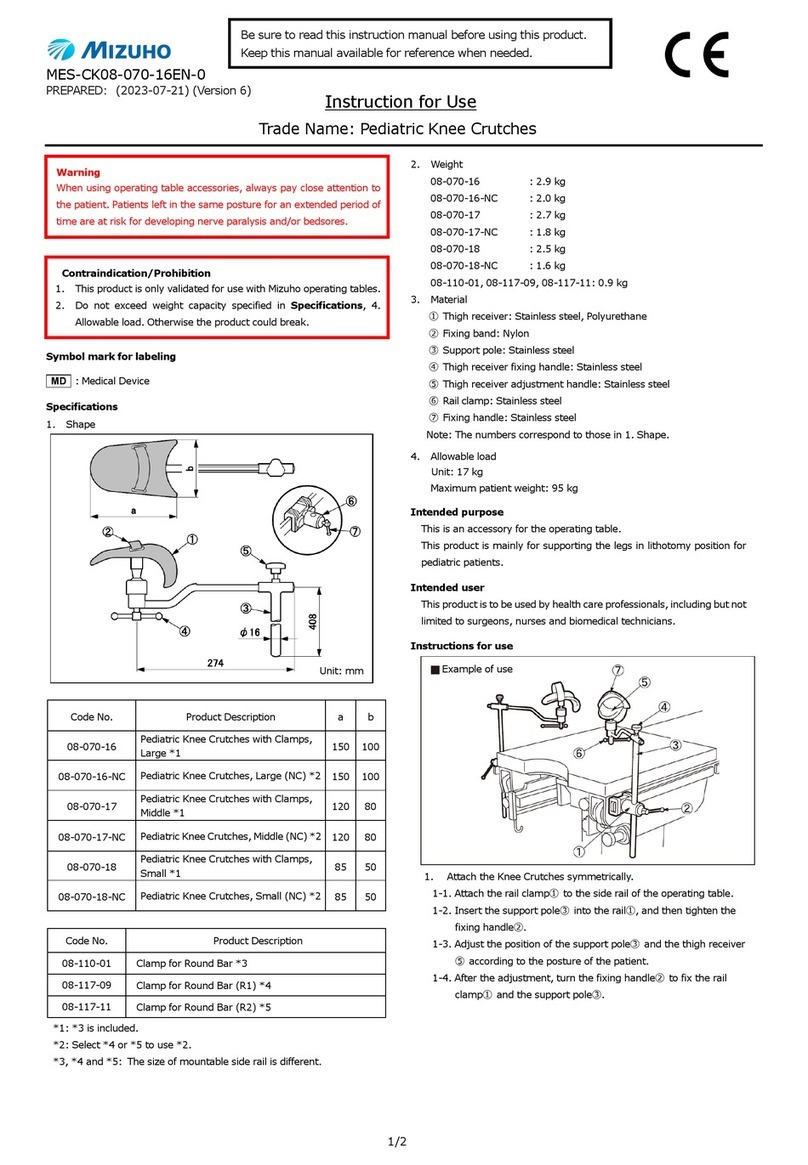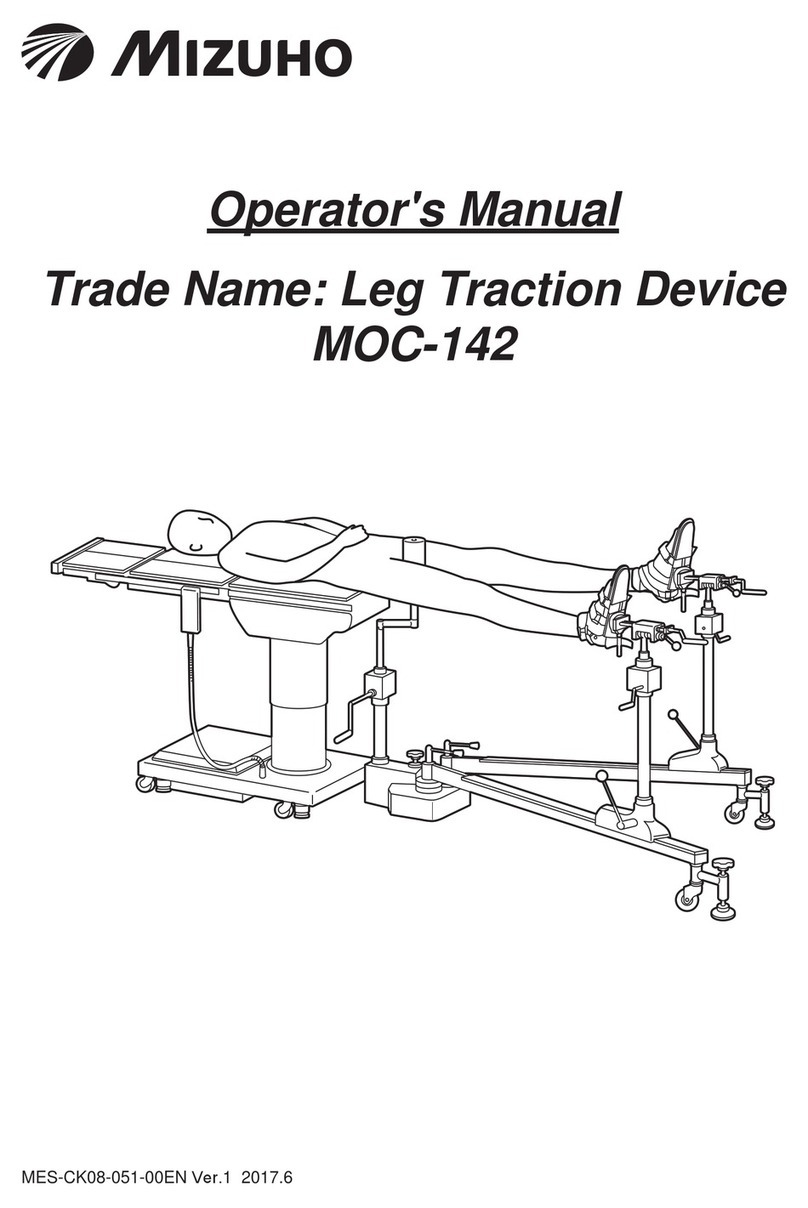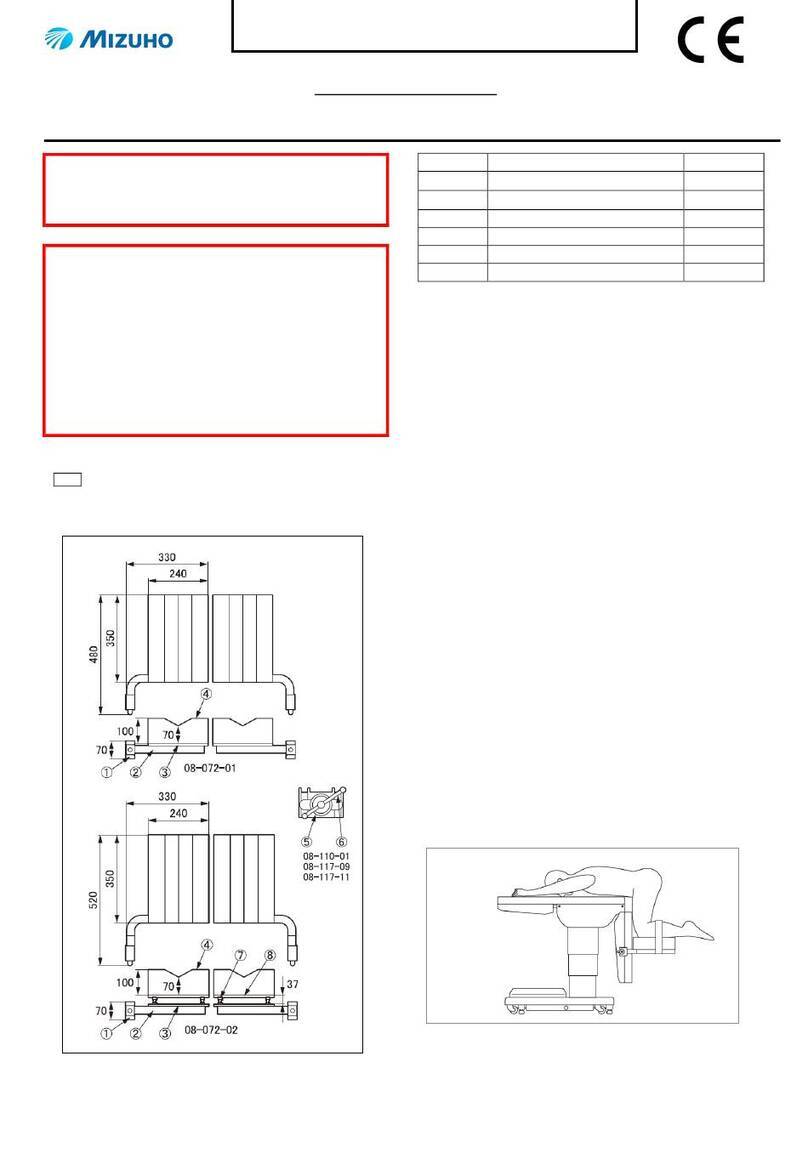
1/2
MES-CK07-870-03CEEN-0
PREPARED: (2018-04-03) (Version 1)
Instructions for Use
Trade Name: DeBakey Micro Forceps
Warning
Please read these instructions carefully prior to using this
product. Product should be used according to these
instructions and pay close attention to the safety of
patients. Not doing so may give rise to serious problems
or adverse events.
For the US market
Do not reuse the device when it is used on a patient with
or suspected of having Creutzfeldt-Jakob Disease (CJD)
or variant CJD (vCJD).
For the markets outside the US
When the device is used on a patient with or suspected of
having CJD or vCJD, make sure to adhere to the most
recent and updated restrictions available in each country
and/or region for its reuse. Be cautious of the possibility
of secondary infection. Refer to www.a-k-i.org or AAMI
Standard ST79 for more information related to cleaning
and sterilization.
Contraindication / Prohibition
1. Use for intended purpose only
Incorrect use can cause breakage of this product. Never
use as a lever or twist the unit with force. Doing so may
cause failure of the internal mechanism or otherwise
damage the instrument.
2. Prohibition of use of chemicals
Avoid exposing this product to chemicals. Doing so could
damage this product due to corrosion.
3. Prohibition of secondary processing of this product
Do not apply any secondary processing to this product.
For example, do not apply impacts or vibration markings
to the surface of this product. Doing so could break this
product.
4. Handle with care
Handle this product with care, as it can be deformed or
damaged. Rough handling could significantly reduce the
service life of devices and appliances.
5. Prohibition of use of polishing powder and wire wool
When cleaning this product, do not attempt to polish its
surfaces with rough polishing powder or wire wool. This
could cause scratches on the surface of this product and
result in rust or corrosion.
6. Prohibition of use of household detergents
Use only medical detergents to clean this product. Do not
use any household detergent. Washing this product with
an improper detergent could result in discoloration or
corrosion.
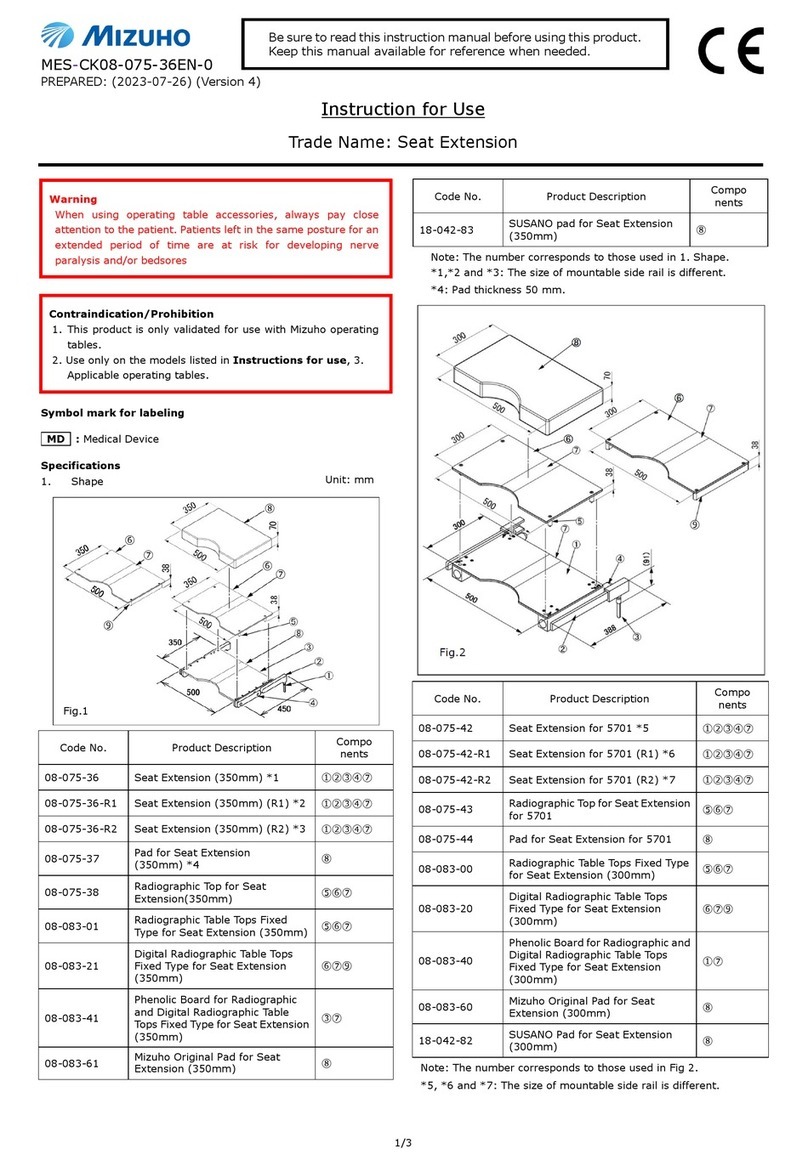
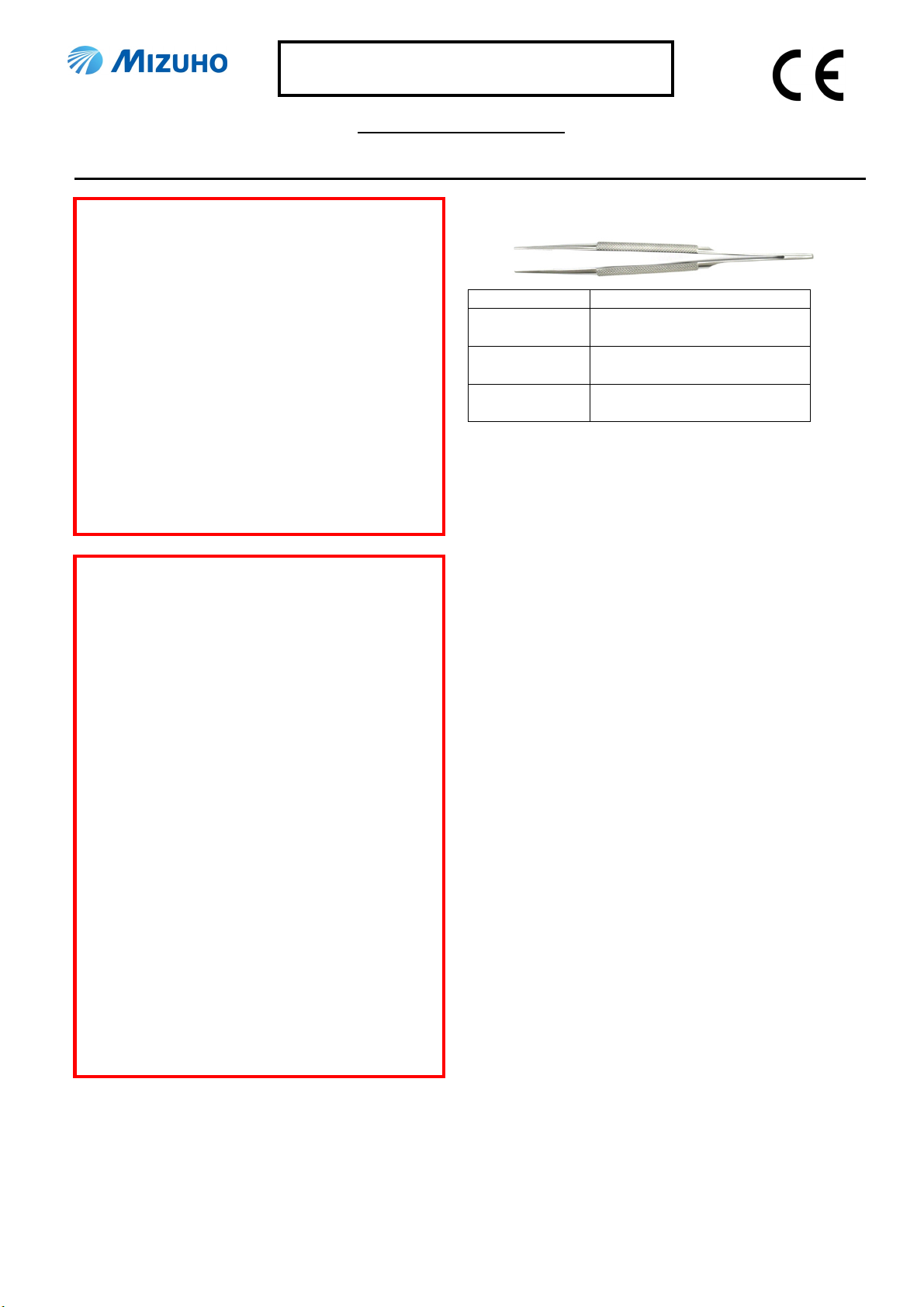
Shape/Structure
Code No. Product Description
07-870-03CE Micro Forceps 15cm, 1.5mm
DeBakey Jaw RH CE
07-870-04CE Micro Forceps 18cm, 1.5mm
DeBakey Jaw RH CE
07-870-05CE Micro Forceps 21cm, 1.5mm
DeBakey Jaw RH CE
Material: Stainless steel
Intended Purpose
This device is a surgical instrument to grasp an object with
the 2 blades placed symmetrically.
Instructions for use
Before using this product, inspect, wash, and sterilize in
accordance with these instructions (See the requirements
relating to maintenance and inspection).
Cautions/Warnings
1. Warning
Device must be sterilized by users in accordance with
sterilization procedures or the validated sterilization
conditions which validity is proven by medical organizations
in each country or region.
2. Defect/Adverse event
Defect
-Corrosion or pitting caused by use of chemicals
-Damage or breakage caused by the corrosion or pitting
-Scissors may not cut properly due to damaged tip of the
blades.
Adverse event
-Broken pieces of metal from the damaged instrument
falling into the patient.
Storage/Life
1. Please store the device in normal ambient temperature
areas. Do not store in areas of high humidity where the
temperature may dramatically vary causing condensation.
Do not store on or near chemicals as the chemicals may
cause damage to the device.
2. Service life of this product: 7 years
(Subject to following manufacturer's specified
maintenance, inspection and proper storage
requirements)
Maintenance/Inspection
1. Check prior to each use
Operational and functional checks
Conduct daily and pre-operation checks of this product to
make sure that it functions properly.
Be sure to read this instruction manual before using this product.
Keep this manual available for reference when needed.