Table of Contents
1.
General Safety Statements ................................................................................................................................. 3
1.1 Summary of Safety Notices: Warnings, Cautions and Notes..................................................................... 3
1.1.
EMC Statement ......................................................................................................................................... 3
1.2.
Electrical Safety Statement ....................................................................................................................... 7
1.3.
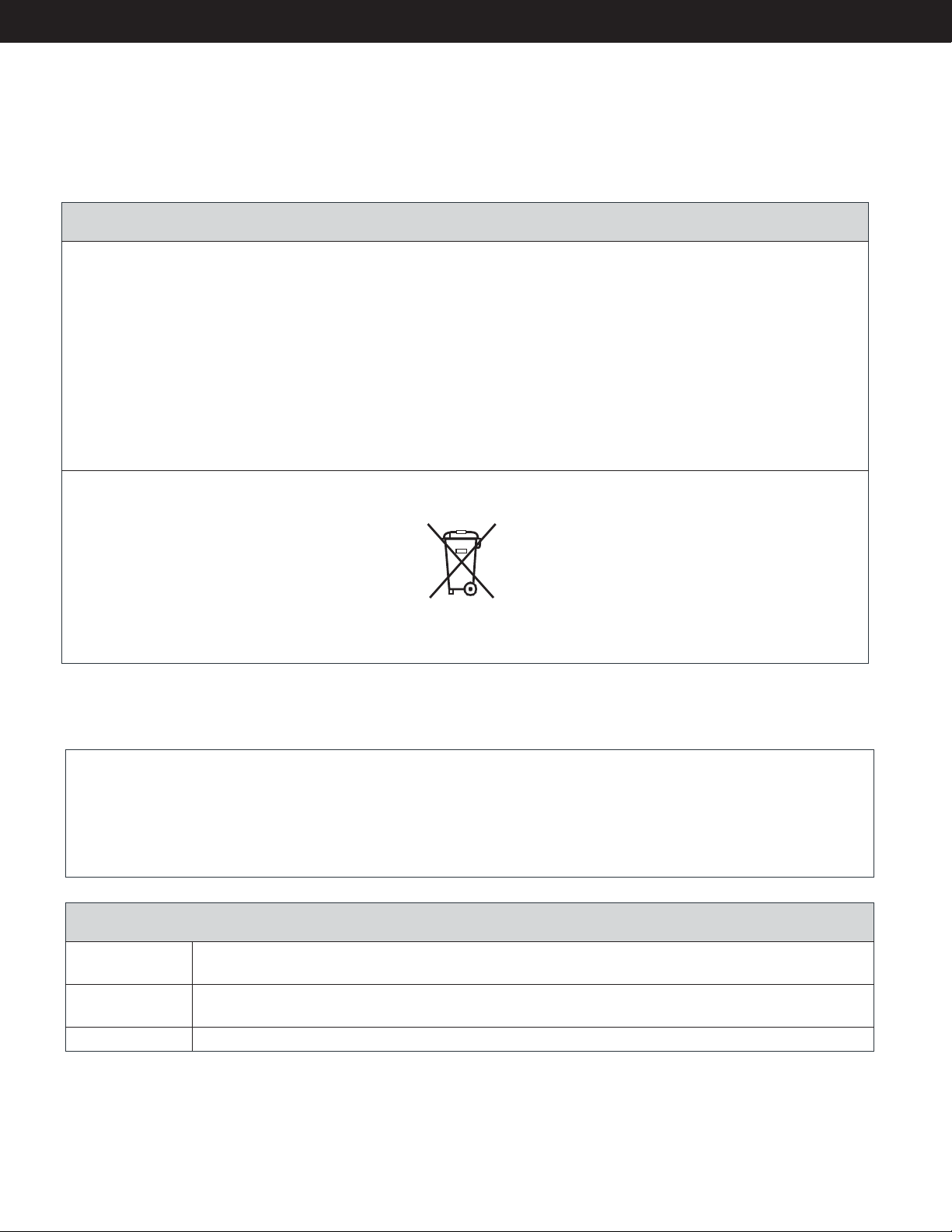
Environmental Statement......................................................................................................................... 8
1.4.
Summary Of Safety Notices ...................................................................................................................... 8
1.5.
Trademark Information............................................................................................................................. 12
1.6.
Explanation Of Symbols ............................................................................................................................ 13
2.
Indications And Contra Indications..............................................................................................................14
2.1.
Indications................................................................................................................................................. 14
2.2.
Contra Indications..................................................................................................................................... 14
3.
Adverse Effects ................................................................................................................................................... 14
4.
Considerations During Clinical Use..............................................................................................................15
4.1.
SonicOne O.R Use...................................................................................................................................... 15
5.
System Overview ................................................................................................................................................ 17
5.1.
Principle Of Operation .............................................................................................................................. 17
5.2.
Reusable System Components...........................................................................................................18
5.3.
Single Use, Sterile Components .........................................................................................................19
6.
Console........................................................................................................................................................19
6.1.
Receptacles, Controls And Indicators ....................................................................................................... 19
6.2.
Menu Functions ........................................................................................................................................ 21
6.3.
Main Functions.......................................................................................................................................... 23
6.4.
Alerts And Indicators................................................................................................................................. 25
7.
System Set-Up..................................................................................................................................................... 27
7.1.
Installation ................................................................................................................................................ 27
7.2.
Console Set-up –Part I (Non-sterile)......................................................................................................... 29
7.3.
Handpiece Assembly (Sterile) ................................................................................................................... 29
7.4.
Console Set-up –Part II (Non-sterile)........................................................................................................ 30
7.5.
Perform System Check .............................................................................................................................. 32
8.
Handpiece Assembly And Disassembly .......................................................................................................32
8.1.
Handpiece Assembly..........................................................................................................................32
8.2.
Handpiece Disassembly .....................................................................................................................33
9.
Cleaning And Sterilization............................................................................................................................34
9.1.
Disassembly .............................................................................................................................................. 34
9.2.
Cleaning .............................................................................................................................................35
9.3.
Sterilizing By Steam Autoclave...........................................................................................................38
9.4.
Expected Life, Reusable Components....................................................................................................... 39
9.5.
Deviations From Decontamination, Cleaning And Sterilization Instructions......................................39
10.
Troubleshooting ..........................................................................................................................................39
11.
Specifications ...................................................................................................................................................... 42
12.
Service, Repair AndTechnical Correspondence ..........................................................................................43
12.1.
Fuse Replacement..................................................................................................................................... 43
12.2.
Pump Head Replacement ..................................................................................................................45
12.3.
Repair, Service and Replacement Parts .................................................................................................... 46
12.4.
Important Notice ...................................................................................................................................... 47