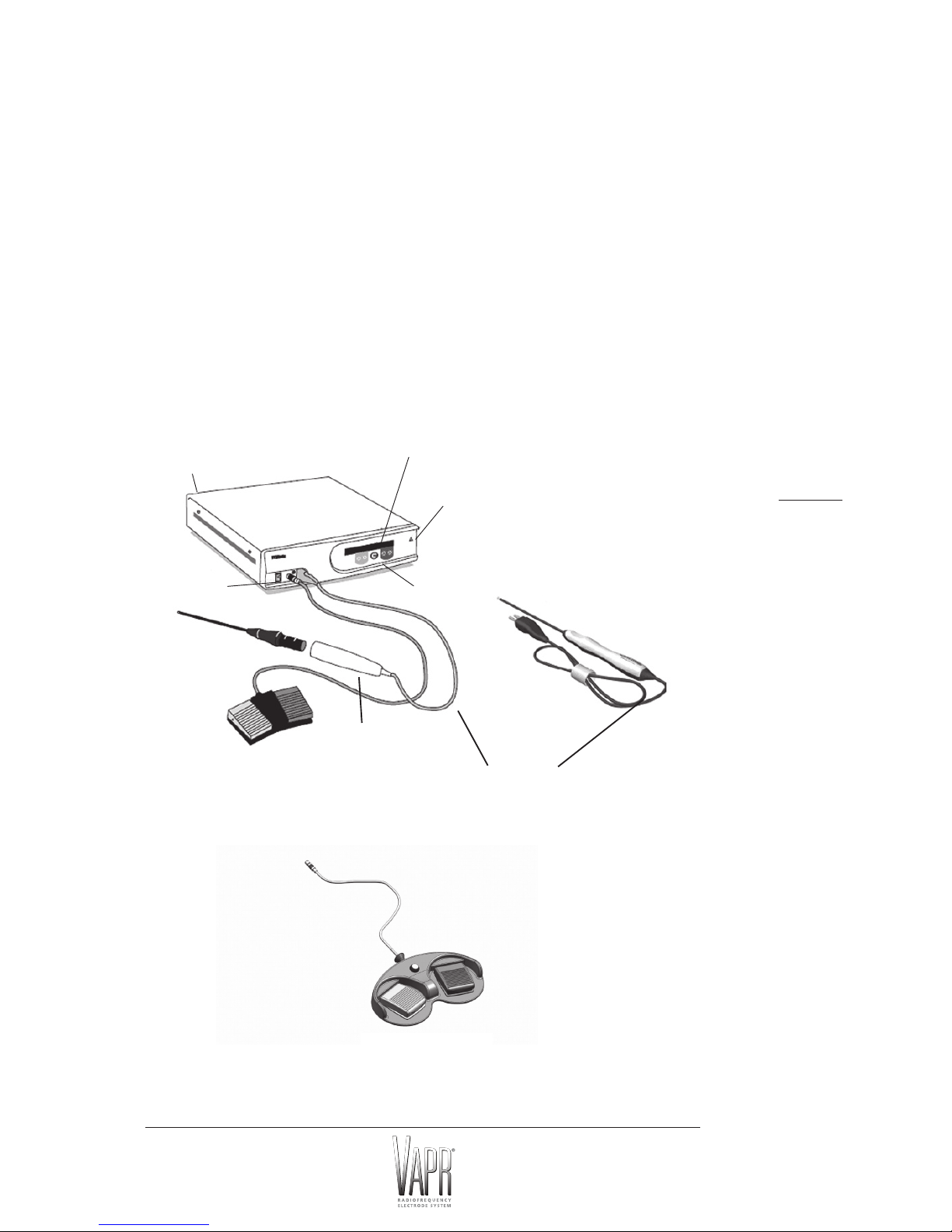
BACKGROUND
Arthroscopy relates to the use of an arthroscope to visualize the joint space. A variety of
instruments specifically designed for arthroscopic use may be introduced through separate
puncture sites, employing the technique of triangulation, in order to perform various surgical
procedures within the joint space.
Arthroscopic instruments have been developed to provide specific functions such as tissue
removal, cutting, shaping and coagulation. Until recently, these instruments have broadly taken
one of three forms; manual instruments, powered instruments, and electrosurgical instruments,
each with respective merits and limitations. As a result, it is common practice to employ a
combination of instruments during an arthroscopic procedure.
The DePuy Mitek VAPR System represents a new and versatile approach to arthroscopy.
Based on an innovative form of bipolar electrosurgery, the VAPR System has been specifically
designed to provide a range of arthroscopic surgical modalities including soft tissue ablation
(electro-vaporization), contouring, cutting and coagulation and temperature indication.
COMPARISON TO CONVENTIONAL ELECTROSURGERY
Conventional electrosurgical systems deliver high frequency electrical current through tissue
for the purposes of tissue cutting or hemostasis of blood vessels. Monopolar electrosurgery
utilizes an “active” electrode located on the surgical instrument and a separate “return”
electrode applied to the patient. Current flow is from the active electrode, through the patient to
the return electrode. Bipolar electrosurgery differs in that both the active and return electrodes
are located on the surgical instrument, thus minimizing the amount of tissue involved in the
electrical circuit.
Problems potentially encountered when using conventional bipolar electrosurgery include
limited power delivery and visualization of the working tip, tissue sticking, and dependence
upon proper electrode-to-tissue orientation. Additionally, conventional bipolar electrodes do not
operate effectively while immersed in conductive irrigating solutions used in arthroscopy, such
as normal saline or Ringer’s lactate.
In contrast, VAPR bipolar electrosurgery Electrodes are specifically designed to function in
conductive irrigating solutions. The VAPR “return” electrode is mounted on the shaft of the
instrument and does not have to be oriented to be in contact with tissue during use. This
eliminates the need for a separate patient ground electrode. Additionally, since only the tissue
that is in contact with the active electrode is involved in the electrical circuit, the recognized
safety features of bipolar electrosurgery are preserved.