2
Introduction and Overview
Excess unincorporated, non-radioactive label can cause high background uorescence
in automated sequencing gels. For optimal sequencing results, remaining labeled
dideoxynucleotides should be removed prior to electrophoresis.
Omega Bio-tek’s Mag-Bind® SeqDTR™ is designed to eectively and reliably remove
unincorporated terminators from sequencing reactions. The system combines Omega
Bio-tek’s proprietary chemistries with the reversible nucleic acid-binding properties
of magnetic beads to eliminate excess nucleotides, primers, and small, non-targeted
amplication products such as primer dimers. This kit is designed for both manual and
fully automated purication of sequencing products.
The Mag-Bind® SeqDTR™ magnetic particles technology provides a better solution
for nucleic acid purication than centrifugation and vacuum-based technologies. The
product can be easily scaled up while providing simple user-friendly handling procedures.
If using the Mag-Bind® SeqDTR™ for the rst time, please read this booklet to become
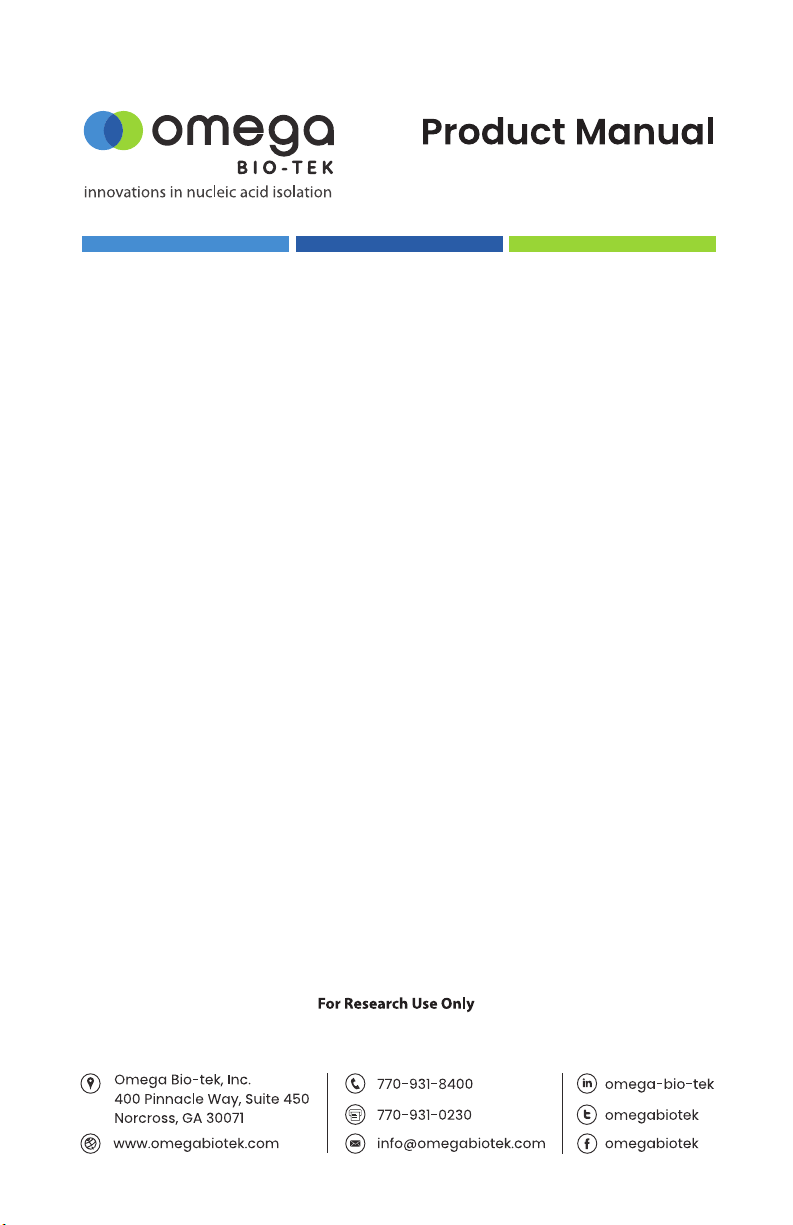
familiar with the procedures. Sequencing products are mixed with the Mag-Bind®
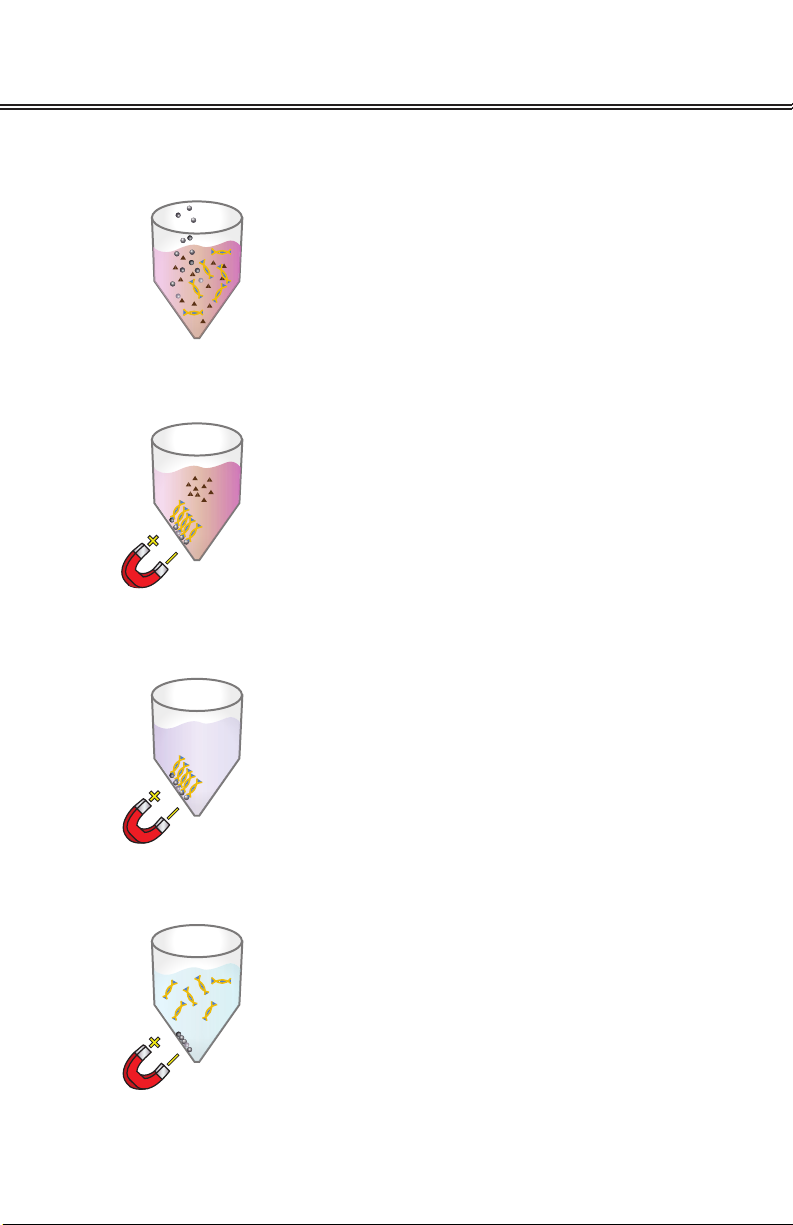
SeqDTR™. DNA selectively binds to the Mag-Bind® SeqDTR™ particles. With one rapid
wash step, trace contaminants such as nucleotides, primers and small, non-targeted
amplication products are removed. Pure DNA is eluted in low salt buer or water.
Puried DNA can be directly used in downstream applications without the need for
further purication.
New in this edition:
• The manual has been updated to reect the product name change from the old
name of Mag-Bind® SE DTR to the new name of Mag-Bind® SeqDTR™.