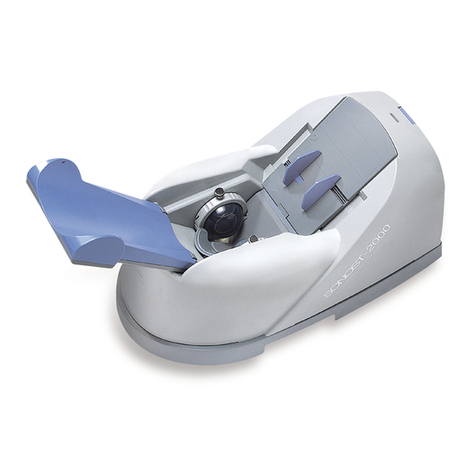
OsteoSys SONOST 3000 User manual

SONOST 3000
User’s Manual
Model: SONOST 3000
Council Directive 93/42/EEC Concerning Medical Device
http://www.osteosys.com
OsteoSys Co., Ltd.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
2
SONOST 3000
User’s Manual
Manufacturer and EC Authorized Representative Information
♣ Manufacturer: OsteoSys Co., Ltd.
901-914, 9F, JnK Digitaltower, 111 Digital-ro 26, Guro-gu, Seoul
152-848, Republic of Korea
Tel: +82 26124 5900 Fax:+82 26124 5958
♣ European Representative: Finlink
Myllärintie 10/76 00920 Helsinki Finland
Tel: +358 44 511 5324
Fax: +358 9 222 3533
CAUTION!
1. You must be well acquainted with this manual before using it.
2. This manual should be placed where the user could read it whenever necessary.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
3
Thank you for purchasing SONOST 3000 Ultrasound Bone Densitometer.
To ensure safe operation and long-term performance stability, it is essential
that you fully understand the functions and operating, maintenance
instructions by reading this manual before operating the equipment.
Particular attention must be paid to all warnings, cautions and notes
incorporated herein.
Incorrect operation, or failure of the user to maintain the equipment
relieves the manufacturer or his agent of the system’s noncompliance with
specifications or of responsibility for any damage or injury.
The following conventions are used throughout the manual to denote
information of special emphasis.
WARNING !
“Warning”is used to indicate the presence of hazard that can cause
severe personal injury, death or substantial property damage if the
warning is ignored.
CAUTION !
“Caution”is used to indicate the presence of hazard that will or can
minor personal injury and property damage if the caution is ignored.
NOTE !
“Note”is used to notify the user of installation, operation or
maintenance information that is important but not hazard related.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
4
The symbols which are shown in this manual or SONOST 3000
The noticed information which should be concerned with
explanation in this manual
The noticed information when the device is operated
The reference page or section
Applied Part Type B
I and O on power switch represent ON and OFF, respectively
The Attention symbol that marks warning and important
information in the user’s manual
The conductor provides a connection between equipment
and the potential equalization bus-bar of the electrical
installation
The date of manufacture
This symbol indicates “caution” for the hot surface.
User Manual
User of the product to check how to check the product..
Communication Status
It indicates communication status of the equipment.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
5
The protecting device from external electromagnetic wave. 1)
This device can be affect from external electromagnetic wave which is related to precision and
operation. When you using this device, we strongly recommend you, avoid from other device from
protect electromagnetic wave.
Disposal of device.2)
This symbol which is printed on the product manual or box means you do not regret as just waste
from home. If you would like to dispose this device, you should send to some place for reuse
electrical device. It will helpful for environment and human’s healthy life. The reuse of this material
is good for save natural resources If you want to more detail things about disposal of device, ask to
manufacturer or distributor.
Cautionary Notes
(1) Environmental Condition
To prevent possible performance loss or malfunction of device components caused by
sudden and excessive environmental changes, as well as the resultant shortening of
their life cycle, the environmental conditions below must be met.
- Working Temperature : Within 10 ~ 40℃
- Working Humidity : Within 30~ 75%
- Air Pressure : Within 700 ~ 1060hPa
(2) Pre-operation Checklist
- Check the switch connection and polarity indicator status, and verify the device
works properly.
- Check all cable connections for their accuracy and safety.
- Double-check the areas that make direct contact with the patient.
- Check the device and the patient for any anomaly.
- If anomaly is found with the device or the patient, take appropriate actions including
suspending device operation under safe conditions.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
6
(3) Cautions for Storage
- Do not apply excessive force when connecting or disconnecting cables.
- Auxiliary devices should be maintained clean, in working conditions.
- Devices should be positioned in their proper positions, so as not to interfere with
worker or patient movement.
(4) Cautions regarding specialist prescription
This device is designedfor osteoporosis diagnosis, and therefore should be used for
the purpose of diagnosis performed by doctors. Its use must be prescribed and
managed by specialists.
Guidance and manufacturer’s declaration
Guidance and manufacturer’s declaration – electromagnetic emissions
The model SONOST-3000 is intended for use in the electromagnetic environment specified below. The
customer or the user of the model SONOST-3000 should assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The model SONOST-3000 uses RF energy only for its
internal functions. Therefore, its RF emissions are very
low and are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class A
The model SONOST-3000 is suitable for use in all
establishments including domestic and those directly
connected to the public low-voltage power supply network
that supplies buildings used for domestic purposes.
Harmonic
emissions
IEC 61000-3-2
Class A
Voltage
fluctuations/
flicker emissions
IEC 61000-3-3
Complies
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
7
Guidance and manufacturer’s declaration – electromagnetic immunity
The model SONOST-3000 is intended for use in the electromagnetic environment specified below.
The customer or the user of the model SONOST-3000 should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment –
guidance
Electrostatic
discharge
(ESD)
IEC 61000-4-2
6 kV contact
8 kV air
6 kV contact
8 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative
humidity should be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1 kV for
input/output lines
2 kV for power
supply lines
1 kV for
input/output lines
Mains power quality should be that of
a typical commercial or hospital
environment.
Surge
IEC 61000-4-5
1 kV differential
mode
2 kV common
mode
1 kV differential
mode
2 kV common
mode
Mains power quality should be that of
a typical commercial or hospital
environment.
Voltage dips,
short
interruption,
and voltage
variations on
power supply
input lines
IEC 61000-4-
11
< 5 % UT
(> 95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
< 5 % UT
(> 95 % dip in UT)
for 5 s
< 5 % UT
(> 95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
< 5 % UT
(> 95 % dip in UT)
for 5 s
Mains power quality should be that of
a typical commercial or hospital
environment. If the user of the model
SONOST-3000 requires continued
operation during power mains
interruptions, it is recommended that
the model SONOST-3000 be
powered from an uninterruptible
power supply or battery.
Power
frequency
(50/60 Hz)
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields
should be at levels characteristic of a
typical location in a typical
commercial or hospital environment
Note : UT is the a.c. mains voltage prior to application of the test level.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
8
Guidance and manufacturer’s declaration – electromagnetic immunity
The model SONOST-3000 is intended for use in the electromagnetic environment specified below.
The customer or the user of model SONOST-3000 should assure that it is used in such an
environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment - guidance
Conducted
RF
IEC61000-
4-6
3 Vrms
150 kHz to
80MHz
3 Vrms
150 kHz to
80MHz
Portable and mobile RF communications equipment
should be used no closer to any part of the model
SONOST-3000 , including cables, than the
recommended separation distance calculated from the
equation applicable to the frequency of the transmitter.
Recommended separation distance
d=[3.5/V1]√P
d=[3.5/E1]√P 80MHz to 800MHz
d=[7/E1]√P 800MHz to 2.5GHz
where P is the maximum output power rating of the
transmitter in watts(W) according to the transmitter
manufacturer and d is the recommended separation
distance in meteres(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,a should
be less than the compliance level in each frequency
range.b
Interference may occur in the vicinity of equipment
marked with the following symbol:
Radiated
RF
IEC61000-
4-3
10 V/m
80MHz to
2.5 GHz
10 V/m
80MHz to
2.5 GHz
Note 1 At 80MHz and 800MHz, the higher frequency range applies.
Note 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects and people.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
9
Safety considerations about part of device and compartment. 3)
For use safely, you must use part of device which supplied or approved from Osteosys.
NOTE !
If you use part of device which is not approved from Osteosys, we do not guarantee safety to user.
Moreover, it become dangerous and errors and wrong results to users or patients. In this case, user has
responsibility.
1) Protect device from external electromagnetic wave (IEC60601-1, 6.8.2 Requirement)
2) Disposal of device (IEC60601-1, 6.8.2 Requirement)
3) Safety considerations about part of device and compartment
(IEC60601-1,6.8.2 Requirement)
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
10
The exchange and using period of expandable goods
Name
Part number
The period for
exchange
The method of exchange
expandable products
Foot supporter
FOOT SUPPORTER 0 :
A3MM-013
10years
You should require to
manufacturer or distributor where
can repair this device.
FOOT SUPPORTER 1 :
A3MM-014
10years
FOOT SUPPORTER 2 :
A3MR-005
10years
FOOT SUPPORTER P :
A3MR-105
10years
QC Phantom
C3MR-004
10years
Power Cable
250VAC 10A
10years
Input and output parts which are using for operate device.4)
Number
Name
Detailed specification
1
paper for printing
Thermal paper,Thickness is over : 0.07mm
2
External printer
It required WindowsXP driver
3
External monitor
It requires 800x600 of resolution and supplied
VGA port
4
USB memory stick
It requires above USB 1.1
5
External mouse
It supplied USB or PS/2
6
External mouse
It supplied USB port
6) Input and output parts which are using for operate device.
(IEC60601-1 contents of test 6.8.2. Requirement

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
11
Table of Contents
Chapter 1. Introduction................................................................................................................13
Chapter 2. Configuration of Device.............................................................................................15
2.1 Composition of Device...................................................................................................15
2.1.1 Shape and Components of Device......................................................................15
2.1.2Accessories List ...............................................................................................16
2.1.3 Articles List ......................................................................................................17
Chapter 3. Using SONOST 3000................................................................................................20
3.1. Daily Test.......................................................................................................................20
3.1.1 Preparation ..........................................................................................................20
3.1.2 Daily Test Procedure............................................................................................21
3.2 Shutting down processing..............................................................................................24
3.2.1 Position of shutdown button................................................................................ 25
3.2.2 Shutting down processing ................................................................................. 26
3.3 The analysis of how to rotate the screen.......................................................................27
3.3.1 The location of screen rotation button ............................................................... 27
3.3.2 The method and sequence order of the screen rotation button ......................... 28
3.3 Using H/W......................................................................................................................29
3.3.1 Patient Measurement Procedure.........................................................................29
3.3.2 Selection of the foot supporter.............................................................................30
3.3.3 Where and how much apply to the patient's heel ............................................. 31
3.3.4 Correct position for patient's foot & body .......................................................... 32
3.3.5 OSD (Auto screen alignment) ........................................................................... 33
3.3.6 Setting of Internal Printer.....................................................................................33
3.4 Using S/W ......................................................................................................................35
3.4.1 Progress Table of Program..................................................................................35
3.4.2 Execution of Program ..........................................................................................36
3.4.3 Setting of the Incipient Environment....................................................................37
3.4.4 Measurement of Bone Mineral Density................................................................43
3.4.5 Revision and Deletion of Patient’s Information....................................................53
3.4.6 Inquiry and Deletion of Clinical History of Existing Patients ................................50
3.4.7 DailyTest of SONOST 3000.................................................................................55
3.4.8 Printing of clinical history and Result...................................................................57
3.4.9 Customize the logo in printing .............................................................................60

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
12
3.4.10 The function of Shot Count ................................................................................61
3.4.11 Setup the worklist ............................................................................................ 62
3.4.12 Setup and sending function of PACS .............................................................. 63
3.5 The method of Data manager S/W ............................................................................... 68
3.5.1 Program operation process .................................................................................68
3.5.2 Start to program ................................................................................................ 69
3.5.3 Program setup ................................................................................................... 70
3.5.4 Backup .............................................................................................................. 71
3.5.5 Patient search and delete ................................................................................. 75
3.5.6 Searching, erasing and printing the result about patients' disease history ....... 78
Chapter 4. Maintenance and Repair of SONOST 3000..............................................................80
4.1 Resolution to Problems..................................................................................................80
4.2 Maintenance and Repair................................................................................................81
4.2.1 Cleaning, Disinfection, Sterilization. ....................................................................81
4.2.2 Product’s life ........................................................................................................82
4.2.3 Stockpile and Replacement of Articles ................................................................82
4.2.4 Storage ................................................................................................................82
4.3 Safe use of SONOST 3000 ......................................................................................... 83
4.3.1 Safety rules ....................................................................................................... 83
4.3.2 Cautions related to use electronic medical equipment ..................................... 84
Chapter 5. Specifications & Software updates ......................................................................... 88
5.1 Specifications .............................................................................................................. 88
5.2 Software updates ........................................................................................................ 90
Chapter 6. Reference..................................................................................................................91
6.1 Definition of Parameter Terminology..............................................................................91
6.2 Reference Data..............................................................................................................93
Chapter 7. Product Warranty .................................................................................................... 9 4

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
13
Chapter 1. Introduction
Osteoporosis is one of the serious diseases. This device is a bone densitometer
which estimates a bone mineral density of the calcaneus by ultrasound.
The measurement site is the calcaneus. It takes about 1 minute to measure
the density and to display the shape of ultrasonic wave by computing
simulation on the monitor.
To ensure safe operation and long-term performance stability, it is essential
that you fully understand the functions, operating and maintenance
instructions reading this manual before operating the equipment.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
14
※Features and Cautions !
- This device is an ultrasound bone densitometer which has precision error
as follows. (Estimated index in vivo)
T-score -0.5 or more : BQI(C.V.%) 5
T-score -0.5 or less : BQI(C.V.%) 2
- The equipment must be operated only by, or under supervision of a qualified
person.
- When you give the appropriate commands in SONOST 3000 software,
ultrasonic waves are generated. The generated ultrasonic waves pass through
the Patient’s calcaneus and the electric signal is treated by SONOST 3000
algorithm.
- All operators must understand the potential hazards in the use of medical
electronic devices. They must be able to recognize hazards and protect them-
selves and others from injury.
- The system should be placed at least 20cm from the wall.
- Never remove any system covers.
- In case of changing the printer for other products, check them according to
IEC/EN60601-1-1.
- Unplug the power cord after using.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
15
Chapter 2. Configuration of Device
2.1 Composition of Device
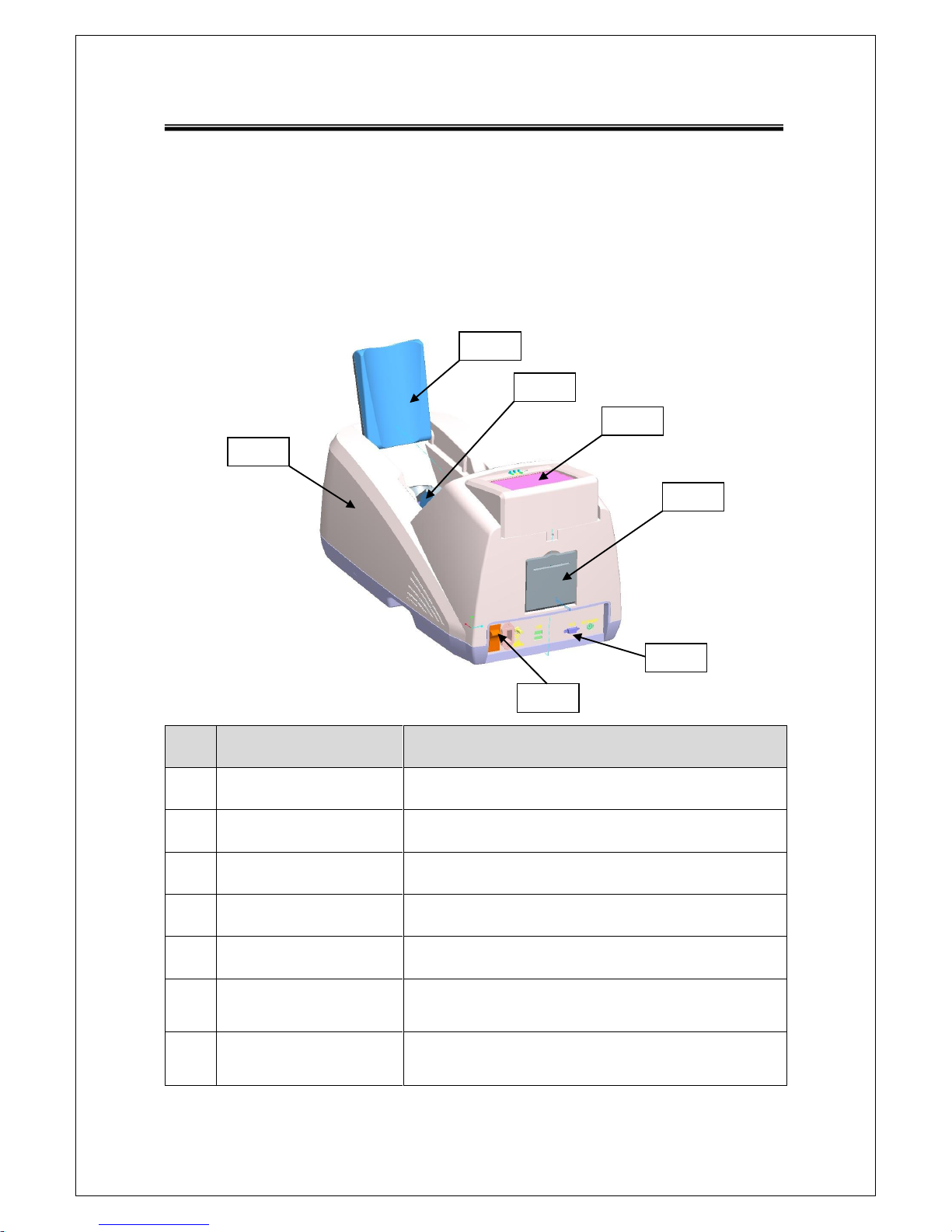
2.1.1 Shape and Components of Device
NO
Name
Function
1-1
TOP COVER
Protection for internal circuit
1-2
CALF SUPPORTER
Leg fixation for diagnosing
1-3
PROBE
Generation of ultrasound
1-4
LCD
Display for measuring status
1-5
THERMAL PRINTER
Thermal printing for the measurement report
1-6
SWITCH PANEL
Connection for the power cord and power ON/OFF
switch
1-7
EXTERNAL
CONNECTOR
USB Port for printer, keyboard and mouse
1-2
1-3
1-4
1-1
1-5
1-7
1-6
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
16
NOTE !
Use external device which certificated by standard (IEC-60601-1).
NOTE !
Use Accessory which defined at user‘s manual.
2.1.2 Accessories List
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its
authorized dealer for this service.
Names of
Products
Number
Uses
User’s Manual
1
Make sure to keep it at a designated place so that
users can read it anytime they need to.
Foot supporter
4
Use them in accordance with patients’foot size.
( base, 0, 1, 2, 3(P) )
QC Phantom
1
It is used to calibration the system.
Keep it in the specified place and protect
it against deformation by heat or
pressure.
Power Cable
1
It is used to connect to the source of electric power
with the main body of SONOST 3000.
Make sure to connect it with a grounded
electric outlet.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
17
2.1.3 Articles List
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its authorized
dealer for this service
4.2.3 Stockpiles and Replacement ofArticles
Names of Products
Number
Uses
Ultrasound Gel
2 bottles
It helps ultrasound to progress between a
probe and patient’s heel.
Alcohol
1 box
It removes some material which prevents
ultrasound from progressing.
Printer Paper
2 rolls
Result paper is printed at printer paper.
Minimum paper thickness : 0.07mm

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
18
2.2 Installation of SONOST 3000
NOTE !
Since SONOST3000is made up of precise components, you should install
the product according to instructions below.
Do not install and keep SONOST3000 at
excessively high or low temperatures.
Proper temperature:
18~27 ℃
Do not install or keep SONOST3000 in the
place where the machine can be rocked or
shaken. Make sure to place the system
parallel to the
floor.
Do not install the system in the place with
polluted air and high humidity and do not
expose the machine to direct ray sunlight. It is
advisable to use SONOST3000 in the place
equipped with air
conditioning or heating.
Proper humidity:
20~80%
Install SONOST3000 in the place where it
is free from water or chemicals.
Do not share the power outlet with other
products through an extension cord.
For escaping from electronic noise, do not
install near a electronic generator, X-ray
equipment, broadcasting equipment.
It causes a result to
be inaccurate.
Do not cover ventilation of the system or place
it near the wall. High inside temperature of the
machine might cause a fire.
100-240V is usable. When SONOST3000 is
taken out of a warehouse, insulating
transformers should be set to 100-240V.
Since sudden power outage can remove all
data saved in the product, you should install
SONOST3000 in the
place where a power
supply is stabilized.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
19
2.3 Before Turning on Power
Connection of Power Cable(with the outlet)
CAUTION !
- Make sure that the covering of cable is
not damaged to prevent electric shock or
short circuit.
- If you find any damage or any sign of it,
immediately contact an agency.
- Put the Power cord into only oneplug.
- Make sure not to share the power outlet
to prevent SONOST 3000 from being
affected.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0423-06-MUL
OT06-2R0423-06 A4(210 ×297)
20
Chapter 3. Using SONOST 3000
3.1. Daily Test
The SONOST 3000 system is checkedusing the daily test.
The daily test should be performed at least once a day, before patient’s measurements.
3.1.1. Preparation
SONOST 3000 device, Phantomand ultrasound transmission gel
Dailytest Recommendation
The daily test should be performedwithin operating temperature.
(Recommended temperature : 18 ~ 27 ℃)
The phantom should be kept beside of device closely.
Daily test should be done just after turning on the device.
Turn on the device and then do Dailytest immediately at beginning time
of day before warming up the device.
Turn off the device after using at closing time of day.
Other manuals for SONOST 3000
2
Table of contents
Other OsteoSys Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Dräger Medical
Dräger Medical Babytherm 8004 Instructions for use

Medi-Globe
Medi-Globe ENDO-FLEX ZB0100 Instructions for use

Ossur
Ossur DIRECT SOCKET TOOL KIT Instructions for use

Jorvet
Jorvet J0563N User manua
Cisco
Cisco TelePresence VX Clinical Assistant Installation and user guide

Lightmed
Lightmed LightLas 532 Operator's manual