OsteoSys SONOST 3000 User manual

SONOST 3000
User’s Manual
Model: SONOST 3000
Council Directive 93/42/EEC Concerning Medical Device
http://www.osteosys.com
OsteoSys Co., Ltd.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
2
SONOST 3000
User’s Manual
Software Version : 3.03.06
Document Version : 4.00
Document Number :
OT06-2R0422-00-MUL
Manufacturer and EC Authorized Representative Information
♣Manufacturer : OsteoSys Co., Ltd.
3F, 308, Byucksan Digital Valley3rd, 212-13, Guro-Dong, Guro-Gu, 152-848,
Seoul, Korea
Tel : 82-2-2025-1650 Fax : 82-2-2025-2299
♣EC Authorized Representative : Ecornet Medical GmbH
High-t' Part Mainstr. 6c-d,D-45768, Marl, Germany
Tel : 49-2365-92-437-0 Fax : 49-2365-92-437-55
CAUTION !
1. You must be well acquainted with this manual before using it.
2. This manual should be placed where the user could read it whenever
necessary.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
3
Thank you for purchasing SONOST 3000 Ultrasound Bone Densitometer.
To ensure safe operation and long-term performance stability, it is essential
that you fully understand the functions and operating, maintenance
instructions by reading this manual before operating the equipment.
Particular attention must be paid to all warnings, cautions and notes
incorporated herein.
Incorrect operation, or failure of the user to maintain the equipment
relieves the manufacturer or his agent of the system’s noncompliance with
specifications or of responsibility for any damage or injury.
The following conventions are used throughout the manual to denote
information of special emphasis.
WARNING !
“Warning” is used to indicate the presence of hazard that can cause
severe personal injury, death or substantial property damage if the
warning is ignored.
CAUTION !
“Caution” is used to
indicate the presence of hazard that will or can
minor personal injury and property damage if the caution is ignored.
NOTE !
“Note”
is used to notify the user of installation, operation or
maintenance information that is important but not hazard related.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
4
The symbols which are shown in this manual or SONOST 3000
The noticed information which should be concerned with
explanation in this manual
The noticed information when the device is operated
The reference page or section
Applied Part Type BF
I and O on power switch represent ON and OFF, respectively
TheAttention symbol that marks warning and important
information in the user’s manual
The conductor provides a connection between equipment and
the potential equalization bus-bar of the electrical installation
The date of manufacture
This symbol indicates “caution” for the hot surface.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
5
Table of Contents
Chapter 1. Introduction ................................................................................................. 7
Chapter 2. Configuration of Device.............................................................................. 9
2.1 Composion of Device ......................................................................................... 9
2.1.1 Shape and Components of Device........................................................... 9
2.1.2Accessories List....................................................................................... 10
2.1.3Articles List............................................................................................ 1 1
2.2 Installation of SONOST 3000 ......................................................................... 12
2.3 Before Turning on Power ................................................................................ 13
Chapter 3. Using SONOST 3000................................................................................. 14
3.1 Using H/W ........................................................................................................ 14
3.1.1 Patient Measurement Procedure........................................................... 14
3.1.2 Where and How MuchApply to the Patient's heel ............................. 15
3.1.3 Correct Position for Patient's Foot & Body......................................... 16
3.1.4 Setting of Internal Printer………...…………………………………...17
3.2 Using S/W ........................................................................................................ 2 0
3.2.1 Progress Table of Program................................................................... 2 0
3.2.2 Execution of Program........................................................................... 2 1
3.2.3 Setting of the Incipient Environment……………………………..….2 2
3.2.4 Measurement of Bone Mineral Density(BMD Measurement) .......... 2 8
3.2.5 Revision and Deletion of Patient's Information ................................. 3 2
3.2.6 Inquiry and Deletion of Clinical History of Existing Patients .......... 3 3
3.2.7 Calibration of SONOST 3000(DailyTest)............................................ 3 4
3.2.8 Printing of clinical history and Result................................................. 3 6
Chapter 4. Maintenance and Repair of SONOST 3000........................................... 3 8
4.1 Resolution to Problems................................................................................... 3 8
4.2 Maintenance and Repair................................................................................ 3 9
4.2.1 Cleaning, Disinfection, Sterilization .................................................... 3 9
4.2.2 Product's Life......................................................................................... 3 9
4.2.3 Stockpile and Replacement of Articles................................................ 4 0
4.2.4 Storage.................................................................................................... 4 0
4.3 Safe Use of SONOST 3000 ............................................................................. 4 1
4.3.1 Safety Rules............................................................................................ 4 1
4.3.2 Cautions Related to Use Electronic Medical Equipment .................. 4 2
Chapter 5. Specifications & Software Updates...................................................... 4 7

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
6
5.1 Specifications................................................................................................... 4 7
5.2 Software Updates............................................................................................ 4 9
Chapter 6. Reference................................................................................................... 5 0
6.1 Definition of Parameter Terminology ........................................................... 5 0
6.2 Reference Data ................................................................................................ 5 2
Chapter 7 . Product Warranty ................................................................................... 5 3

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
7
Chapter 1. Introduction
Osteoporosis is one of the serious diseases. This device is a bone
densitometer which estimates a bone mineral density of the calcaneus by
ultrasound.
The measurement site is the calcaneus. It takes about 1 minute to measure
the density and to display the shape of ultrasonic wave by computing
simulation on the monitor.
To ensure safe operation and long-term performance stability, it is essential
that you fully understand the functions, operating and maintenance
instructions reading this manual before operating the equipment.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
8
※
Features and Cautions !
-This device is an ultrasound bone densitometer which has ±0.2(T-score)
precision error.
-The equipment must be operated only by, or under supervision of a
qualified person.
-When you give the appropriate commands in SONOST 3000 software,
ultrasonic waves are generated. The generated ultrasonic waves pass
through the Patient’s calcaneus and the electric signal is treated by
SONOST 3000 algorithm.
-All operators must understand the potential hazards in the use of
medical electronic devices. They must be able to recognize hazards and
protect themselves and others from injury.
-The system should be placed at least 20cm from the wall.
-Never remove any system covers.
-In case of changing the printer for other products, check them
according to IEC/EN60601-1-1.
-Unplug the power cord after using.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
9
Chapter 2. Configuration of Device
2.1 Composition of Device
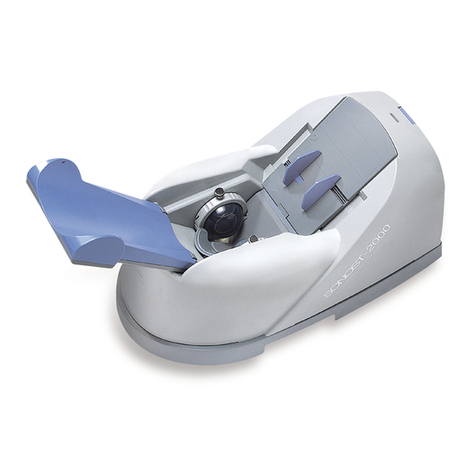
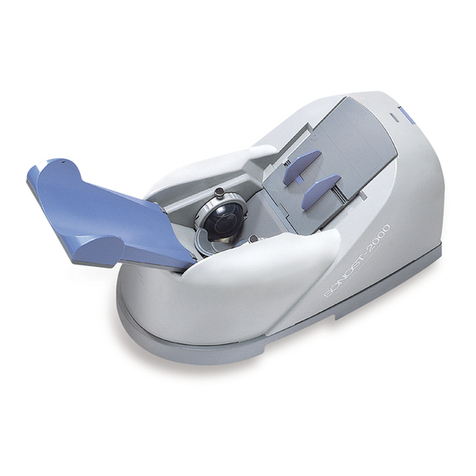
2.1.1 Shape and Components of Device
1-1
1-2
1-3 1-4
1-5
1-6
1-7
N
O Name Function
1-1 TOPCOVER Protection for internal circuit
1-2 CALF SUPPORT Leg fixation for diagnosing
1-3 PROBE Generation of ultrasound
1-4 LCD Display for measuring status
1-5 THERMAL PRINTER Thermal printing for the measurement report
1-6 SWITCH PANEL Connection for the power cord and power
ON/OFF switch
1-7 EXTERNAL
CONNECTOR USB Port for printer, keyboard and mouse

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
10
NOTE !
Use external device which certificated by standard (IEC-60601-1).
NOTE !
Use Accessory which defined at user‘s manual.
2.1.2 Accessories List
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its
authorized dealer for this service.
Names of
Products Number Uses
User’s Manual 1 Make sure to keep it at a designated place so that
users can read it anytime they need to.
Foot Positioner 3 Use them in accordance with patients’foot size.
QC Phantom 1
It is used to calibration the system.
Keep it in the specified place and
protect it against deformation by heat
or pressure.
Power Cable 1
It is used to connect to the source of electric
power with the main body of SONOST 3000.
Make sure to connect it with a
grounded electric outlet.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
11
2.1.3 Articles List
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its authorized
dealer for this service
4.2.2 Stockpiles and Replacement of Articles
Names of Products Number Uses
Ultrasound Gel 2 bottles It helps ultrasound to progress between a
probe and patient’s heel.
Alcohol 1 box It removes some material which prevents
ultrasound from progressing.
Printer Paper 2 rolls Result paper is printed at printer paper.
Minimum paper thickness : 0.07mm

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
12
2.2 Installation of SONOST 3000
NOTE !
Since SONOST3000 is made up of precise components, you should install the
product according to instructions below.
Do not install and keep SONOST3000 at
excessively high or low temperatures.
Proper
temperature:
18~27
℃
Do not install or keep SONOST3000 in
the place where the machine can be
rocked or shaken. Make sure to place
the system
parallel to the
floor.
Do not install the system in the place with
polluted air and high humidity and do not
expose the machine to direct ray sunlight. It
is advisable to use SONOST3000 in the
place equipped with air conditioning or
heating.
Proper humidity:
20~80%
Install SONOST3000 in the place where
it is free from water or chemicals.
Do not share the power outlet with other
products through an extension cord.
For escaping from electronic noise, do not
install near a electronic generator, X-ray
equipment, broadcasting equipment.
It causes a
result to be
inaccurate.
Do not cover ventilation of the system or
place it near the wall. High inside
temperature of the machine might cause a
fire.
100-240V is usable. When SONOST3000
is taken out of a warehouse, insulating
transformers should be set to 100-240V.
Since sudden power outage can remove
all data saved in the
product, you should
install
SONOST3000 in the
place where a power
supply is stabilized.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
13
2.3 Before Turning on Power
Connection of Power Cable(with the outlet)
CAUTION !
Make sure that the covering of
cable is not damaged to prevent
electric shock or short circuit.
If you find any damage or any
sign of it, immediately contact an
agency.
Put the Power cord into only one
plug
Make sure not to share the power
outlet to prevent SONOST 3000
from being affected.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
14
Chapter 3. Using SONOST 3000
3.1 Using H/W
3.1.1 Patient Measurement Procedure
Clean the surface of the
Coupling Pad with an alcohol and
apply gel enough.
Clean the both side of patient’s
heel
with an alcohol pad and apply
gel.
Insert patient’s foot.
Let a patient move his foot up to
down just like this picture.
Start to measure after insertion.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
15
NOTE !
Insert patient’s foot after widen probe’s gap
enough, because probe
pad is made from soft silicon.
3.1.2 Where and How MuchApply to the Patient’s Heel
Before measuring, using alcohol to rub patient’s heel :
protection for removing air bubbles surface of patient’s skin and other
infections.
Apply plenty of ultrasound gel to both side of patient’s heel
Good
No Good

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
16
3.1.3 Correct position for Patient’s foot & body
Adhere a heel to the measuring device closely as shown 3 points below.
Make sure measuring device and body should be one line as shown below.
Patient
Device

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
17
3.1.4 Setting of Internal Printer
Paper type : Thermal paper width 58mm. thickness : MIN 0.07mm
(About 80 reports are printable by one roll)
Paper character : Only printable in an outer surface
Purchasing : Purchase from the headquarters or an agency.
Paper inserting
①Cover opening : Pull-down open the dark gray cover on the device’s the
back side
②Paper inserting : Insert the paper as shown below.
Outer surface of paper should face to the inside of the
device.
Paper is scrolled in automatically.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
18
③Cover shutting : Put the paper to the gap in the cover, then shut the cover
up.
⑥Paper cutting : For a sharp outline of report printing, cut off the remained
paper once by a saw tooth in the cover.
CAUTION !
Do not touch the metal part of the thermal printer head. The hot
surface may cause to burn.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
19
3.2 Using S/W
3.2.1 Progress Table of Program
Start
Print History Print Result
Home
Patient List
History List
Measure
Result
Dailytest
Setup
Product
Information
Start Measure
Add Patient
Search Patient
Modify Patient
Delete Pateint
Delete History

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation: SONOST 3000 DOC. No. : OT06-2R0422-00-MUL
OT00-2F0423-01 A4(210 ×297)
20
3.2.2 Execution of Program
CAUTION !
If you hear any strange noise from the screen, switch it off and contact to
OsteoSys or its Authorized dealer.
Turn on the power switch.
10 seconds after calibration that checks functions of the system and memory
device, the program is automatically activated and you should configure the
incipient environment.
This is the incipient screen.
Home of the incipient screen
There are Main buttons on the top line, It is used when the screen is moved.
The bottom line is consisted of Sub buttons, it is use for sub function.
Other screens are similar formation.
- QuickMeasure : Direct measuring function without patient’s registration process.
- DailyTest : Daily test function.
When the daily test is needed, the color of button is red.
After it is need not, the color of button is turn to same with others.
- Setup : Consists of functions which are related with program setting.
- Information : Display information about the program and device.
Other manuals for SONOST 3000
2
Table of contents
Other OsteoSys Medical Equipment manuals