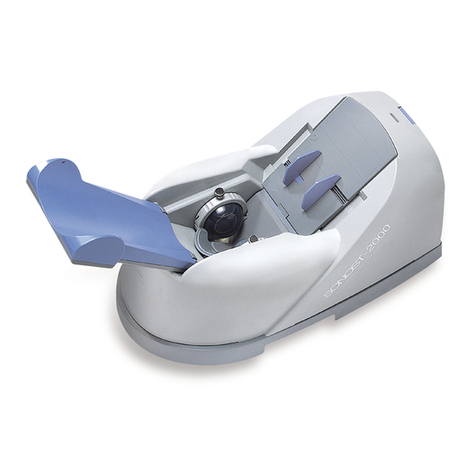
OsteoSys SONOST-2000 User manual

User’s Manual
Model: SONOST-2000
REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENTAND OF THE
COUNCIL
Doc Version : 12.2_MDR (2022.09.05)
http://www.osteosys.com
OsteoSys Co., Ltd.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
2
User’s Manual
CAUTION !
You must be well acquainted with this manual before using it.
This manual should be placed where the user could read it whenever
necessary.
Manufacturer and EC Authorized Representative Information
♣ Manufacturer: OsteoSys Co., Ltd.
901~914, 9F, JnK Digitaltower, 111 Digital-ro 26, Guro-gu, Seoul, REPUBLIC OF KOREA
Tel: +82 26124 5900 Fax:+82 26124 5958
♣ European Representative: CMC Medical Devices & Drugs S.L.
C/Horacio Lengo Nº18, CP 29006, Málaga, Spain
TEL: +34 951 214 054 FAX: +34 952 330 100

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
3
Thank you for purchasing SONOST-2000 Ultrasound Bone Densitometer. To ensure safe
operation and long-term performance stability, it is essential that you fully understand the
functions and operating, maintenance instructions by reading this manual before operating the
equipment.
Particular attention must be paid to all warnings, cautions and notes
incorporated herein.
Incorrect operation, or failure of the user to maintain the equipment relieves the
manufacturer or his agent of the system’s noncompliance with specifications or of
responsibility for any damage or injury.
The following conventions are used throughout the manual to denote information of special
emphasis.
WARNING !
“Warning”is used to indicate the presence of hazard that can cause severe personal
injury, death or substantial property damage if the warning is ignored.
CAUTION !
“Caution”is used to indicate the presence of hazard that will or can minor personal
injury and property damage if the caution is ignored.
NOTE !
“Note”is used to notify the user of installation, operation or maintenance
information that is important but not hazard related.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
4
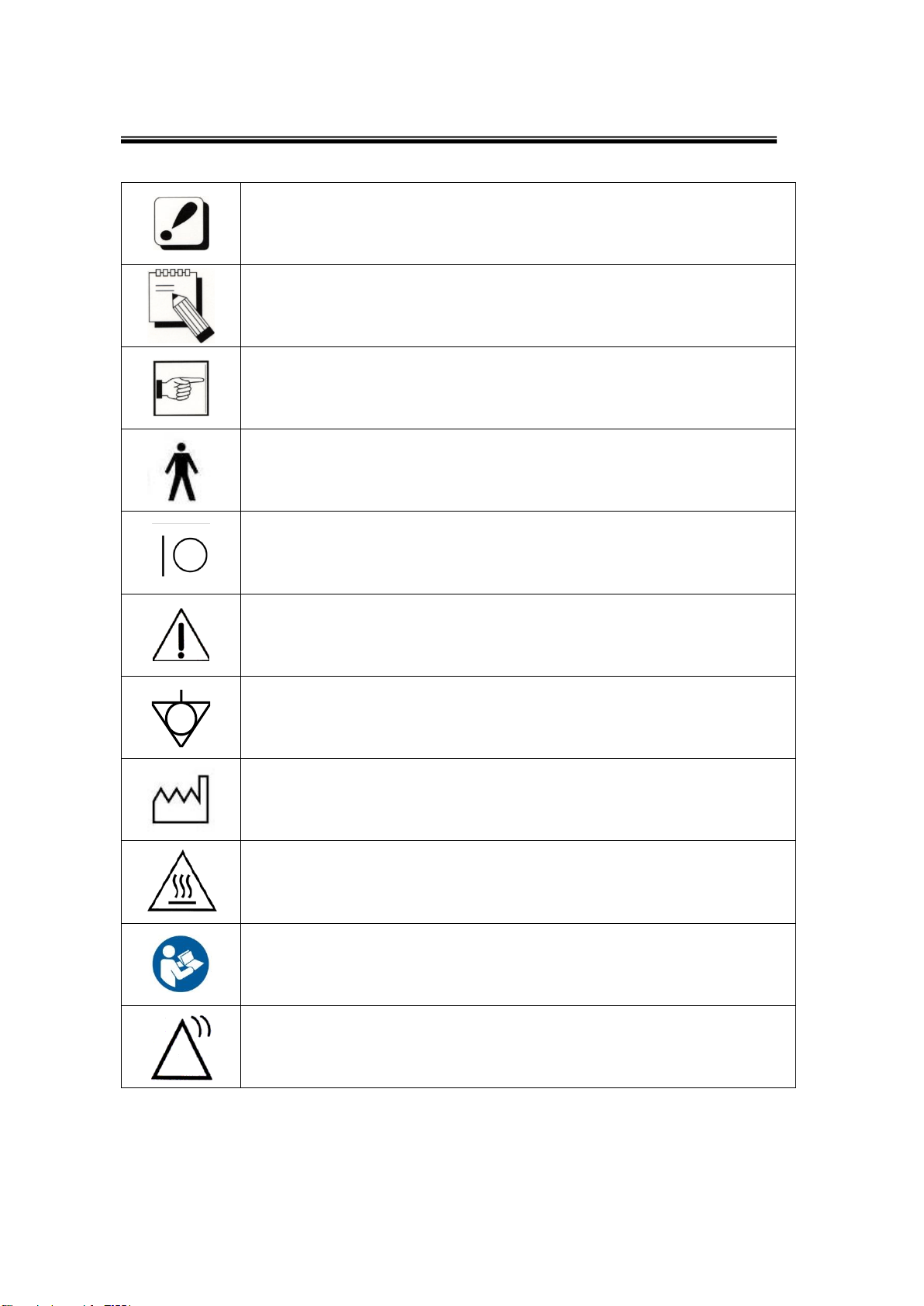
The symbols which are shown in this manual or SONOST-2000
The noticed information which should be concerned with
explanation in this manual
The noticed information when the device is operated
The reference page or section
Applied Part Type B
I and O on power switch represent ON and OFF, respectively
The Attention symbol that marks warning and important
information in the user’s manual
The conductor provides a connection between equipment and the
potential equalization bus-bar of the electrical installation
The date of manufacture
This symbol indicates “caution” for the hot surface.
User Manual
User of the product to check how to check the product..
Communication Status
It indicates communication status of the equipment.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
5
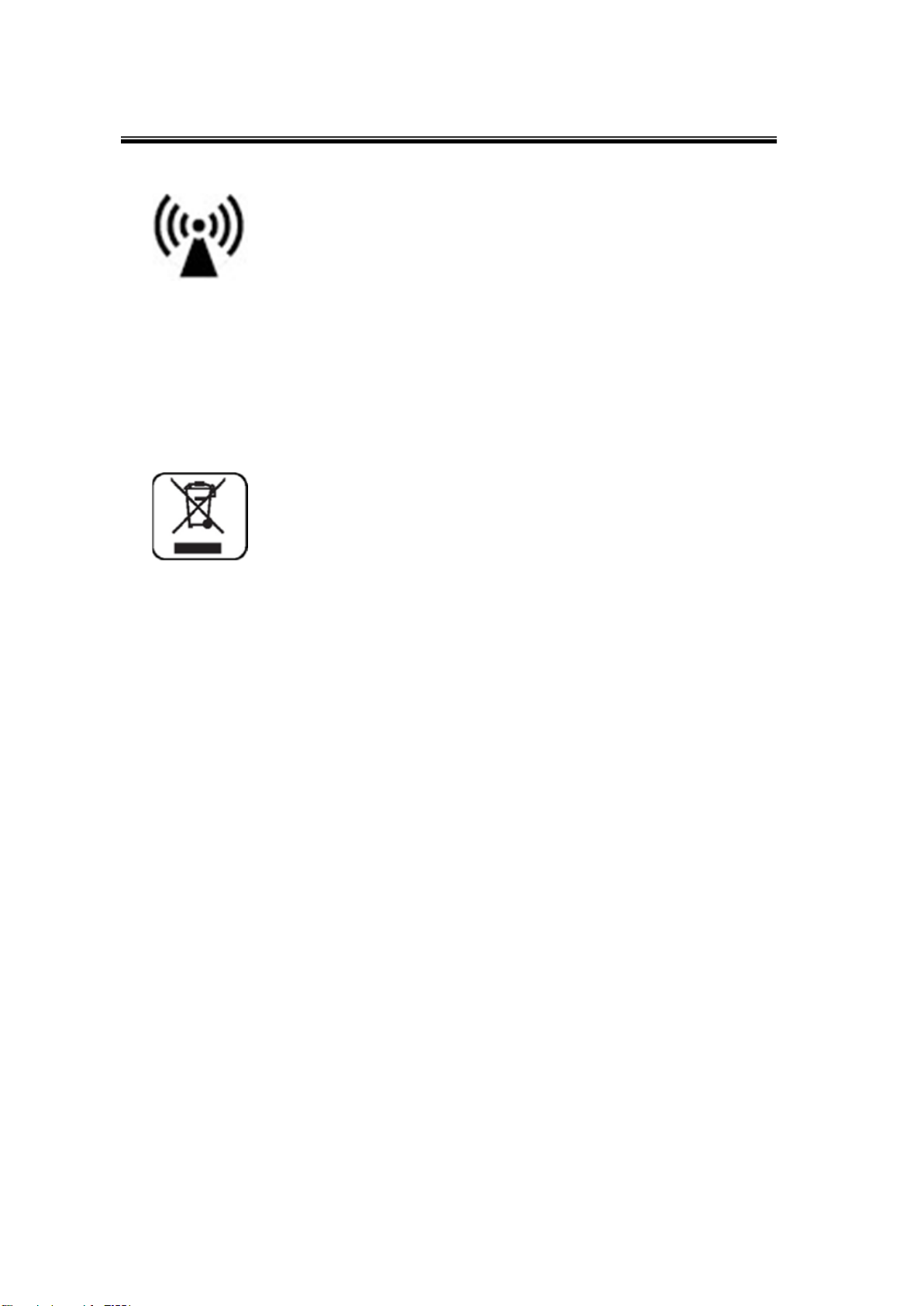
The protecting device from external electromagnetic wave
This device can be affect from external electromagnetic wave which is related to precision and operation.
When you using this device, we strongly recommend you, avoid from other device from protect
electromagnetic wave.
Disposal of device
This symbol which is printed on the product manual or box means you do not regret as just waste from
home. If you would like to dispose this device, you should send to some place for reuse electrical device.
It will helpful for environment and human’s healthy life. The reuse of this material is good for save natural
resources If you want to more detail things about disposal of device, ask to manufacturer or distributor.
Cautionary Notes
(1) Environmental Condition
To prevent possible performance loss or malfunction of device components caused by sudden and
excessive environmental changes, as well as the resultant shortening of their life cycle, the
environmental conditions below must be met.
- Working Temperature : Within 10 ~ 40℃
- Working Humidity : Within 20~ 80%
- Air Pressure : Within 800 ~ 1060hPa
(2) Pre-operation Checklist
- Check the switch connection and polarity indicator status, and verify the device works properly.
- Check all cable connections for their accuracy and safety.
- Double-check the areas that make direct contact with the patient.
- Check the device and the patient for any anomaly.
- If anomaly is found with the device or the patient, take appropriate actions including suspending
device operation under safe conditions.
(3) Cautions for Storage
- Do not apply excessive force when connecting or disconnecting cables.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
6
- Auxiliary devices should be maintained clean, in working conditions.
- Devices should be positioned in their proper positions, so as not to interfere with worker or patient
movement.
(4) Cautions regarding specialist prescription
This device is designed for osteoporosis diagnosis, and therefore should be used for the purpose of
diagnosis performed by doctors. Its use must be prescribed and managed by specialists.
(5) Biocompatibility - Ultrasound gel and etc
To prevent damage to skin and cells, use commercially available ultrasound gels that have passed ISO
10993-5(Cytoxicity), ISO 10993-10(Skin sensitization) and ISO 10993-23(Skin irritation) tests.
Guidance and manufacturer’s declaration
Guidance and manufacturer’s declaration – electromagnetic emissions
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
7
The model SONOST-2000 is intended for use in the electromagnetic environment specified below. The customer
or the user of the model SONOST-2000 should assure that it is used in such an environment.
No
Contents
A or
N/A
Description
1
Intended Use
A
The SONOST-2000 system supports the doctor's activities for the diagnosis
of osteoporosis and the prevention of future fractures to the patient
through the measured BMD results. The measurement sites is calcaneus/
Heel bone.
2
Name of Disease
or Condition
A
Osteoporosis, Osteopenia, Normal healthy bone
3
Indications
A
All women 65 years and older and men 70 years and older for
asymptomatic screening.
Women younger than 65 years old at risk for osteoporosis:
Estrogen deficiency
History of maternal hip fracture before the age of 50
Low body mass (less than 57.6kg/127 pounds)
History of amenorrhea more than 1 years before the age of 42
Women younger than 65 years old or men younger than 70 years old with
the following risk factors:
Current cigarette smoker
Loss of height
Thoracic Kyphosis
Individuals at any age with bone mass osteopenia or fragility fractures on
imaging studies
Individuals 50 years and older who develop wrist, hip, spine, or proximal
humerus fracture with minimal or no trauma, excluding pathologic
fractures.
People of any age who develop one or more insufficiency fractures.
Individuals receiving (or expected to receive) glucocorticoid therapy
equivalent to > or = to 5 mg of prednisone or equivalent per day for > or
= 3 months.
Individuals beginning or receiving long-term therapy with medications
known to affect BMD adversely:
• Anticonvulsants
• Androgen deprivation therapy
• Aromatase inhibitor therapy
• Chronic heparin
Individuals with an endocrine disorder known to affect BMD adversely
• Hyperparathyroidism
• Hyperthyroidism
• Cushing’s syndrome
Hypogonadal men 18 years and older and men with surgically or
chemotherapeutically induced castration
Individuals with medical conditions that could alter BMD:
• Chronic renal failure
• Rheumatoid arthritis and other inflammatory arthritides
• Eating disorders, including anorexia nervosa and bulimia

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
8
• Organ transplantation
• Prolonged immobilization
• Conditions associated with secondary osteoporosis, such as
gastrointestinal malabsorption or malnutrition, sprue, osteomalacia,
vitamin D deficiency, endometriosis, acromegaly, chronic alcoholism or
established cirrhosis, and multiple myeloma
• Individuals who have had a gastric bypass for obesity
Individuals considering pharmacologic therapy for osteoporosis.
Individuals monitored for:
• Assess the effectiveness of osteoporosis drug therapy
• Follow-up medical conditions associated with abnormal BMD.
Children or adolescents with medical conditions associated with abnormal
BMD including but not limited to:
• Individuals receiving (or expected to receive) glucocorticoid therapy
for more than 3 months
• Individuals receiving radiation or chemotherapy for malignancies
• Individuals with an endocrine disorder known to adversely affect BMD
(e.g., hyperparathyroidism, hyperthyroidism, growth hormone deficiency
or Cushing’s syndrome)
• Individuals with bone dysplasias known to have excessive fracture risk
(osteogenesis imperfecta, osteopetrosis) or high bone density
• Individuals with medical conditions that could change BMD, for
example:
1. Chronic renal failure
2. Rheumatoid arthritis and other inflammatory arthritides
3. Eating disorders, including anorexia nervosa and bulimia
4. Organ transplantation
5. Prolonged immobilization
6. Conditions associated with secondary osteoporosis, such as;
gastrointestinal malabsorption, sprue, inflammatory bowel disease,
malnKKutrition, osteomalacia, vitamin D deficiency, acromegaly, cirrhosis,
HIV infection, prolonged exposure to fluorides
QUS may be indicated in the diagnosis, staging, and follow-up of
individuals with conditions that result in pathologically increased BMD,
such as osteopetrosis or prolonged exposure to fluoride.
4
Contraindications
A
There are no absolute contraindications to performing QUS.
Possibly of limited value or require modification of the technique or
rescheduling of the examination in some situations, including:
- Try to measure the other side of heel if patient had some surgery like
implant which is placed by calcaneus bone.
5
Target
group
User
A
Education: Researcher with knowledge of Bone density
Knowledge: Understands human body and bone density
Language Understanding: English
Experience: The person who have been educated or trained from BMD.
(Patient does not operate the BMD device)
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
9
Patient
A
a) Ages: 20 –100 years old
b) The patient condition: Osteopenia, Osteoporosis or Normal healthy bone
6
Disposable device
A
SONOST-2000 has silicon balloon on the probe part which can keep the
water inside of balloon as media for path of ultrasound wave more
efficiently.
7
Invasive device
N/A
Not intended to be invasive
8
Implantable device
N/A
Not intended to be implantable
9
Duration of use or
contact with body
A
BMD measurement is performed once a year or once every two years
(it’s depending on the patient's situation).
Contact with body:
SONOST-2000: there are contact point between calcaneus part and
balloon as below image.
SONOST 3000: It also same as SONOST-2000
Need to apply ultrasound gel in between skin and probe in order to get
proper contact for path of ultrasound waves.
10
Contacting with
body fluids or
others
N/A
There are no contact between anybody fluid of human during the
examination process.
11
Measurement site
A
Correct position for measurement for calcaneus bone on heel as below
That point is just below area of ankle and then apply ultrasound gel with
enough amount just like below instruction. (need to apply to both side of
feet)
*According to the article Selection of the optimal skeletal site for fracture
risk prediction, the most significant relationships of BMC to fracture risk
were observed for the Oscalcis (Richard D. Wasinch, Philip D. Ross, Lance K.
Heilbrun and John M. Vogel)
*According to the article Diagnostic value of calcaneal quantitative
ultrasound in the evaluation of osteoporosis in middle-aged and elderly
patients, the study assessed calcaneal QUS and DXA in senile osteoporosis
diagnosis performance, as osteoporosis screening not only has good
specific degrees, and with the lumbar spine and proximal femur DXA has
good correlation, in screening for large-scale people play the role of more
convenient, economic, Therefore, calcaneal QUS can be recommended as a
pre-screening tool to determine whether DXA screening should be
performed and timely treatment should be performed I patients with pre-
existing risk of osteoporosis and patients with mild osteoporosis to reduce
the risk of fracture. (Changzhou Li, Jifeng Sun, Li Yu)
Emissions test
Compliance
Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The model SONOST-2000 uses RF energy only for its internal
functions. Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic equipment.
RF emissions
CISPR 11
Class A
The model SONOST-2000 is suitable for use in all establishments
including domestic and those directly connected to the public
low-voltage power supply network that supplies buildings used
for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
10
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Complies

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
11
Guidance and manufacturer’s declaration – electromagnetic immunity
The model SONOST-2000 is intended for use in the electromagnetic environment specified below. The customer
or the user of the model SONOST-2000 should assure that it is used in such an environment.
Immunity
test
IEC 60601
test level
Compliance
level
Electromagnetic environment –guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
6 kV contact
8 kV air
6 kV contact
8 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1 kV for
input/output lines
2 kV for power
supply lines
1 kV for
input/output lines
Mains power quality should be that of a
typical commercial or hospital
environment.
Surge
IEC 61000-4-5
1 kV differential
mode
2 kV common
mode
1 kV differential
mode
2 kV common
mode
Mains power quality should be that of a
typical commercial or hospital
environment.
Voltage dips,
short
interruption, and
voltage
variations on
power supply
input lines
IEC 61000-4-11
< 5 % UT
(> 95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
< 5 % UT
(> 95 % dip in UT)
for 5 s
< 5 % UT
(> 95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
< 5 % UT
(> 95 % dip in UT)
for 5 s
Mains power quality should be that of a
typical commercial or hospital
environment. If the user of the model
SONOST-2000 requires continued
operation during power mains
interruptions, it is recommended that the
model SONOST-2000 be powered from
an uninterruptible power supply or
battery.
Power frequency
(50/60 Hz)
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields should
be at levels characteristic of a typical
location in a typical commercial or
hospital environment
Note : UT is the a.c. mains voltage prior to application of the test level.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
12
Table of Contents
1Chapter 1. Introduction...............................................................................................................................14
1.1 General Product Information...........................................................................................................14
2Chapter 2. Configuration of SONOST-2000...............................................................................................15
2.1 Composition of SONOST-2000.......................................................................................................16
2.1.1 Shape and Components of SONOST-2000.................................................................................16
2.1.2 Keyboard .................................................................................................................................16
2.1.3 Accessories List.......................................................................................................................17
2.1.4 Articles List.............................................................................................................................19
2.2 Installation of SONOST-2000 .........................................................................................................20
2.3 Before Turning on Power ................................................................................................................21
3Chapter 3. Using SONOST-2000................................................................................................................23
3.1 Using H/W.......................................................................................................................................23
3.1.1 Applying the Balloon to the Measuring Device .........................................................................23
3.1.2 Using the QC phantom............................................................................................................25
3.1.3 Patient Measurement Procedure ..............................................................................................26
3.1.4 Disposing water.......................................................................................................................28
3.1.5 Where and how much apply to the Patient’s heel ....................................................................28
3.1.6 Correct position for Patient’s foot & body ..............................................................................29
3.1.7 Selection of the foot supporter.................................................................................................30
3.2 Using S/W .......................................................................................................................................32
3.2.1 Progress Table of Program ......................................................................................................32
3.2.2 Using Program.........................................................................................................................33
4Chapter 4. Maintenance and Repair of SONOST-2000..............................................................................70
4.1 Resolution to Problems ...................................................................................................................70
4.2 Maintenance and Repair..................................................................................................................71
4.2.1 Cleaning ..................................................................................................................................71
4.2.2 Stockpile and Replacement of Articles....................................................................................72
4.2.3 Storage.....................................................................................................................................73
4.3 Safe Use of SONOST-2000.............................................................................................................74
4.3.1 Safety Rules................................................................................................................................74
4.3.2 Cautions Related to Use Electronic Medical Equipment.........................................................75
5Chapter 5. Specifications & Software Updates........................................................................................79
5.1 Specifications ..................................................................................................................................79
5.2 Software Updates ............................................................................................................................80

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
13
5.3 Labels..............................................................................................................................................82
6Chapter 6. Product Warranty.......................................................................................................................82
7Chapter 7. Security .....................................................................................................................................85

1Chapter 1. Introduction
1.1 General Product Information
Osteoporosis is one of the serious diseases. This device is a bone densitometer which
estimates a bone mineral density of the calcaneus by ultrasound.
It takes about 15 seconds to measure the density and to display the shape of ultrasonic
wave by computing simulation on the monitor.
To ensure safe operation and long-term performance stability, it is essential that you
fully understand the functions, operating and maintenance instructions reading this
manual before operating the equipment.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
15
2Chapter 2. Configuration of SONOST-2000
※Features and Cautions !
-This device is an ultrasound bone densitometer which has ±0.2(T-score)
precision error.
-The equipment must be operated only by, or under supervision of a qualified
person.
-When you give the appropriate commands in SONOST-2000 software,
ultrasonic waves are generated. The generated ultrasonic waves pass through
the Patient’s calcaneus and the electric signal is treated by SONOST-2000
algorithm.
-SONOST-2000 measures speed of sound(SOS) and the frequency-dependent
attenuation of the sound waves(BUA), and combines them to form a clinical
measure called Bone Quality Index(BQI)
-All operators must understand the potential hazards in the use of medical
electronic devices. They must be able to recognize hazards and protect
themselves and others from injury.
-The system should be placed at least 20cm from the wall. However the USB
cable length should not be longer than 3.0m.
-Never remove any system covers.
-In case of changing the PC or the printer for other products, check them
according to IEC/EN60601-1-1.
-Unplug the power cord before you move or clean the device and after using
SONOST-2000
-For installation and operation of this equipment national regulation shall be
considered.

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
16
2.1 Composition of SONOST-2000
2.1.1 Shape and Components of SONOST-2000
NO
Name
Function
1-1
TOP COVER
Protection for internal circuit
1-2
BOTTOM PLATE
Fixation for device position & top cover
1-3
SWITCH PANEL
Switch, Power cord, RS-232 Connector
1-4
BALLOON
Connect between heel and ultrasound probe
1-5
CALF SUPPORT
Leg fixation for diagnosing
1-6
FOOT GUIDE
Foot fixation for diagnosing
1-7
STATUS LAMP
Present status of the Device
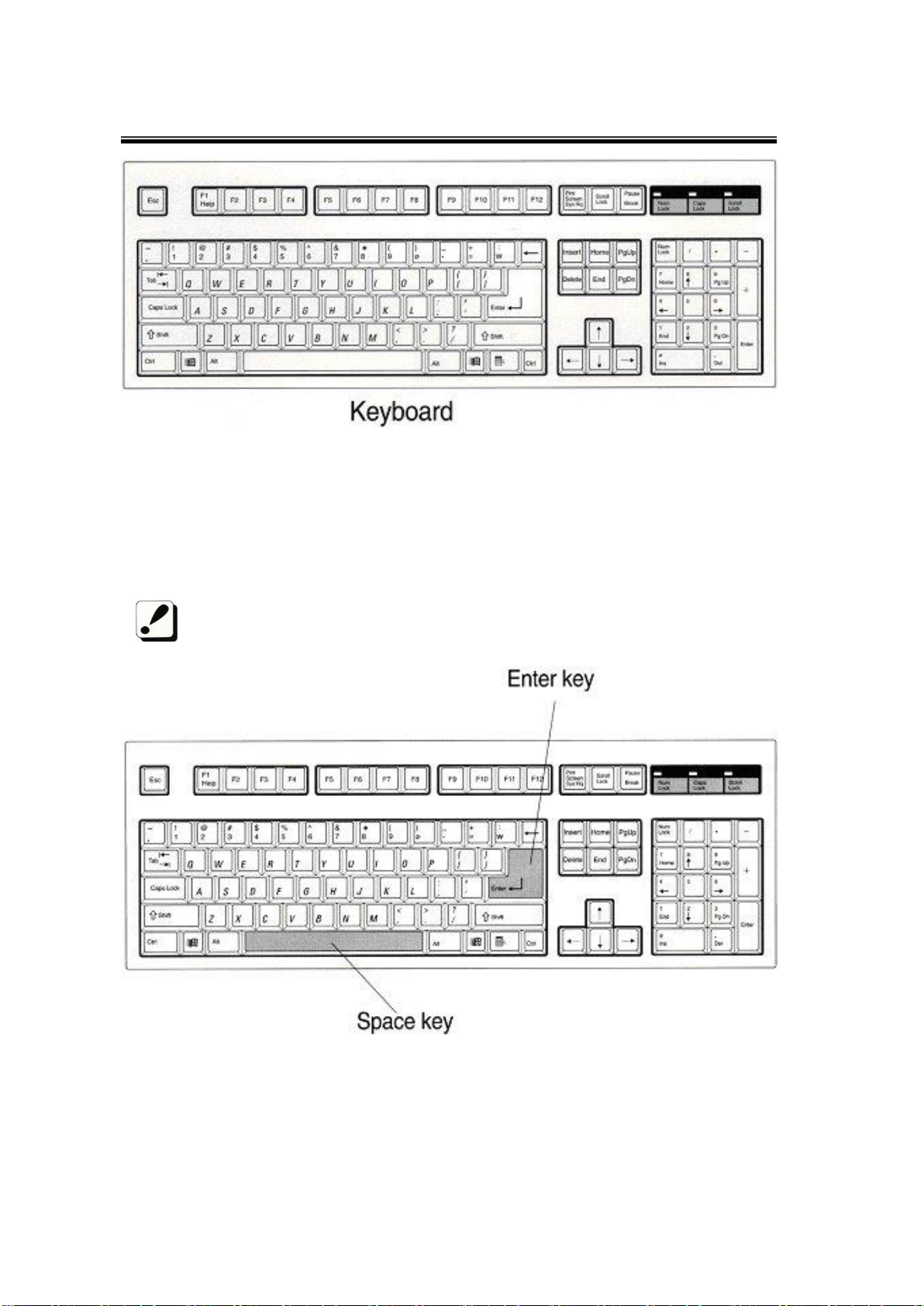
2.1.2 Keyboard
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
17
Activate SONOST-2000 by pressing a key on the keyboard according to the
instructions shown on the monitor.
The manual shows keys required to activate SONOST-2000 as shown below.
2.1.3 Accessories List

User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
18
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its authorized dealer
for this service.
Names of Products
Number
Uses
User’s Manual
1
Make sure to keep it at a designated place so that
users can read it anytime they need to
Foot Positioner
3
Use them in accordance with patients’foot size.
QC Phantom
1
It is used to calibrate the system.
Keep it in the specified place and protect it
against deformation by heat or pressure.
Injector
1
It helps to take water into the balloon easily.
Guide
5
It helps to take water into the balloon easily.
USB Cable
1
It is used to connect to the computer with the main
body of SONOST-2000
Power Cable
1
It is used to connect to the source of electric power
with the main body of SONOST-2000
Make sure to connect it with a grounded
electric outlet.
Program CD
1
It is used to install the program on the
computer.(The program is installed on the
computer before being delivered)
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
19
2.1.4 Articles List
Check the following accessories before installing the system.
If they are not in good condition, contact to OsteoSys or its authorized dealer
for this service.
4.2.2 Stockpiles and Replacement ofArticles
Names of Products
Number
Uses
Balloon / Housing
Balloon : 8
Housing : 1
It connects a foot with the ultrasound probes
and helps ultrasound to progress between them.
Keep the spare balloons and housings in
a cool place.
Alcohol
1 box
It removes some material which prevents
ultrasound from progressing.
User’s Manual (Confidential)
OsteoSys Co., Ltd.
Model Designation : SONOST-2000 DOC. No. : OT01-2R7123
20
2.2 Installation of SONOST-2000
NOTE !
Since SONOST-2000 is made up of precise components, you should install the
product according to instructions below.
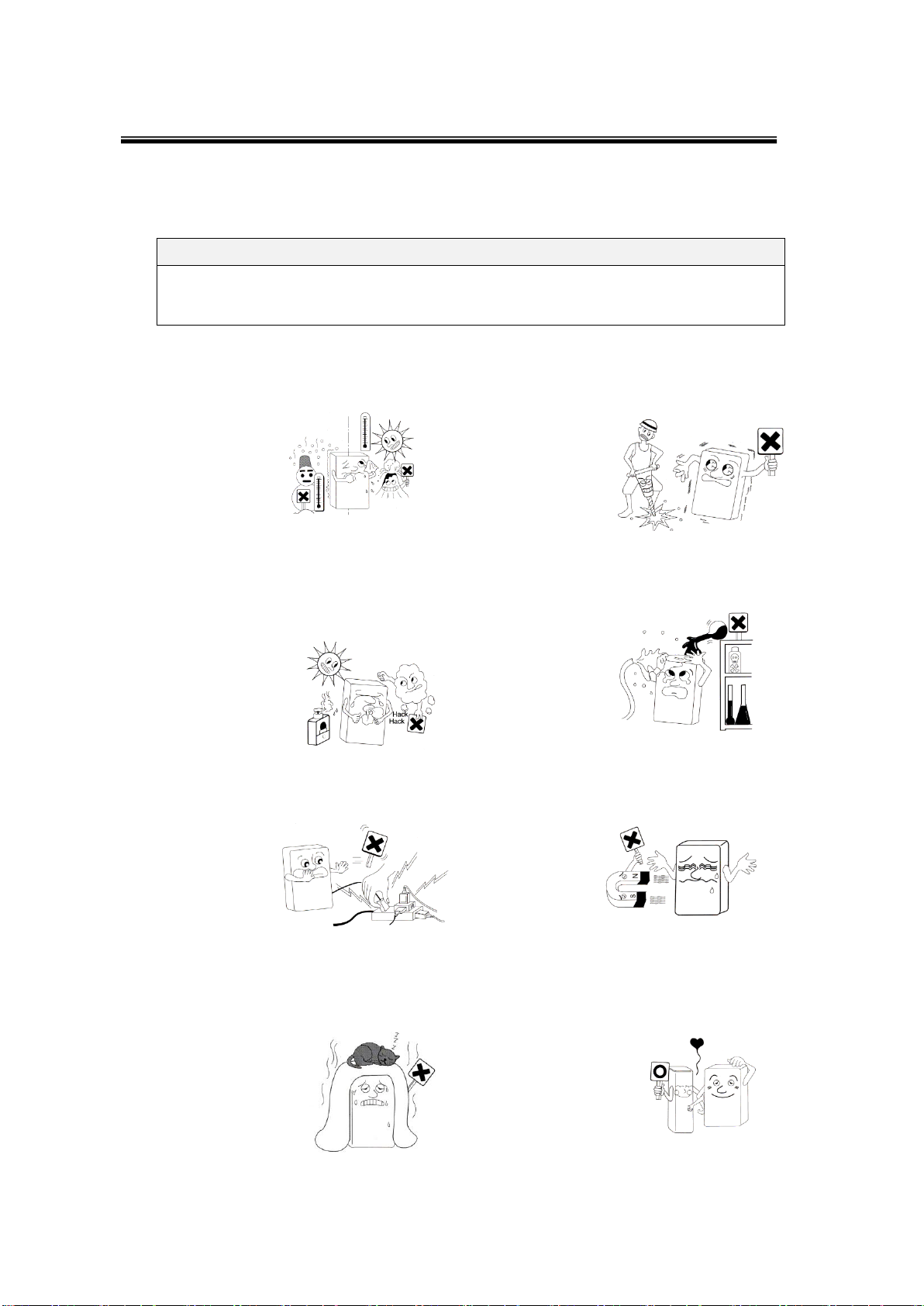
Do not install and keep SONOST-2000 at
excessively high or low temperatures.
Proper
temperature:
18~27 ℃
Do not install or keep SONOST-2000 in
the place where the machine can be
rocked or shaken. Make sure to place
the system
parallel to the
floor.
Do not install the system in the place with
polluted air and high humidity and do not
expose the machine to direct ray sunlight.
It is advisable to use SONOST-2000 in the
place equipped with air conditioning or
heating.
Proper humidity:
20~80%
Install SONOS-2000 in the place where
it is free from water or chemicals.
Do not share the power outlet with other
products through an extension cord.
Do not use the system near equipment
that generates strong magnetic fields.
Do not cover ventilation of the system or
place it near the wall. High inside
temperature of the machine might cause a
fire.
100-240V is usable. When SONOST-
2000 is taken out of a warehouse,
insulating transformers should be set to
100-240V. Since sudden power outage
can remove all data
saved in the product,
you should install
SONOST-2000 in the
place where a power
supply is stabilized.
Other manuals for SONOST-2000
1
Table of contents
Other OsteoSys Medical Equipment manuals
Popular Medical Equipment manuals by other brands

AVONEX
AVONEX AVOSTARTGRIP Instructions for use
Lighting Science
Lighting Science Healthe CLEANSE PORTAL installation instructions

Orthofix
Orthofix Cervical-Stim 2505CE instruction manual

ResMed
ResMed Ultra Mirage NVFFM Clinical Guide

AcuPearl
AcuPearl PAINLESS MkIII user guide

Pensar Medical
Pensar Medical WoundPro Quick reference card