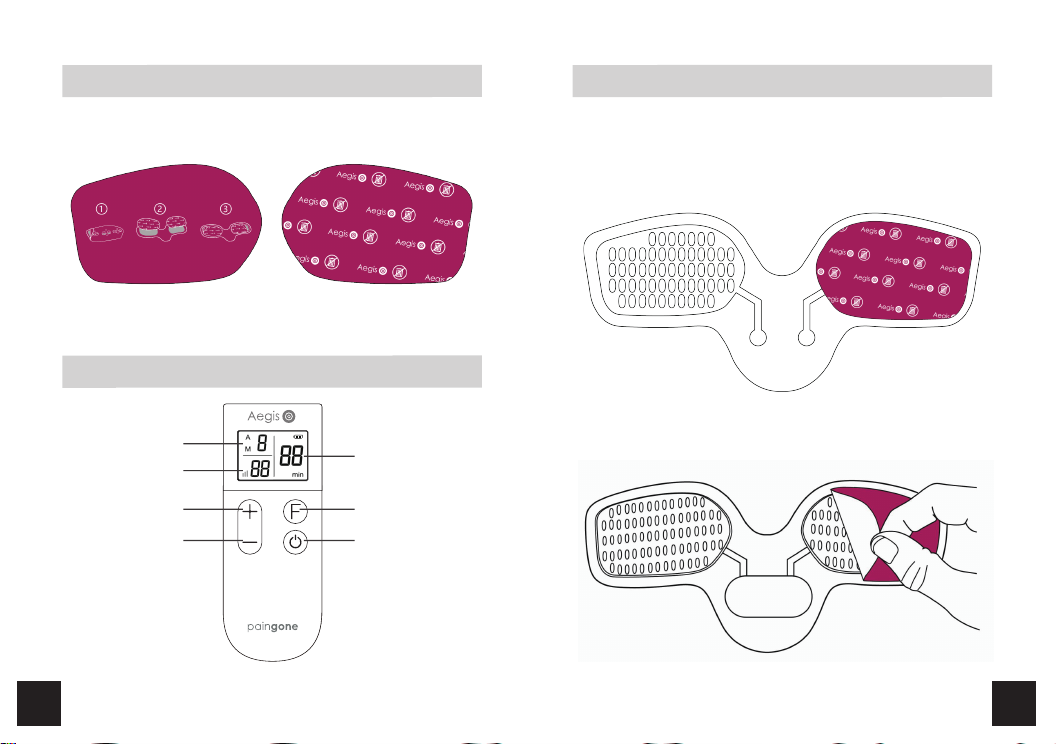
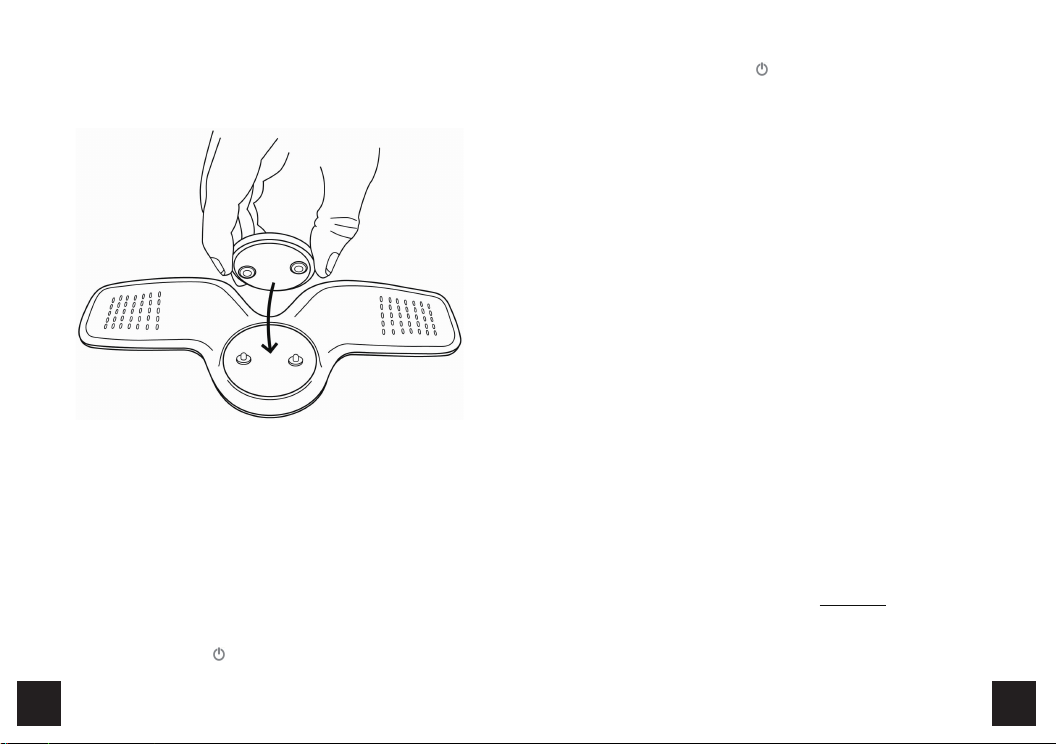
Accessories of Transcutaneous Electronic Nerve Stimulator, Models: KTR-2492
KTR-2491A
14 15
Usage Contraindications
⑴This device is contraindicated for those with a skin perceptual
disturbance or those unable to detect heat
⑵It is prohibited to use when bathing, sweating and sleeping.
⑶It is contraindicated for use by those with a purulent inflammation, acute
blood poisoning or continuous hyperpyrexia.
⑷It is contraindicated for use by those with acute cardiovascular or
cerebrovascular diseases.
⑸Please terminate use immediately and consult with a doctor if you
experience irritation of the skin during use.
⑴Pregnant women and menstruating women, people with sensitive skin,
heart disease, abnormal blood pressure, malignant tumors,
cerebrovascular patients, patients with acute disease or other persons
treated by a doctor must consult a doctor before using this product. ⑴Please do not use over the heart, on the head, eyes, front of neck
(especially the carotid artery), lower back, oral cavity or pudendum, or on
diseased skin
⑴Turn off the device if changing the area of application, otherwise there
is a risk of strong stimulation.
⑵If you feel unwell while using the product, please stop use immediately
and consult with a doctor if necessary
⑶Do not used in conjunction with other medical electronic devices, such
as cardiac pacemaker, artificial heart and lung and other medical
electronic devices such as electrocardiograph.
⑷Do not use the product in strong direct sunlight, or where there is high
heat, inflammable material, electromagnetic radiation and or high humidity.
⑸Do not disassemble, repair or modify the device. This risks damaging
the device and causing electric shock.
⑹The device should be placed in a position that is easily reached in case
it needs to be removed in an emergency
⑺Check the equipment before each use to avoid exposed wires caused
by accidental damage or other reasons.
⑻Dust may affect the performance of the unit, please use a dry soft cloth
to clean the device as needed.
⑼Please check whether the electrode is loose before each use, otherwise
it may have adverse effects on performance or cause other problems.
precautions
-- Simultaneous connection of a patient to high frequency surgical Medical
Electrical (ME) equipment may result in burns at the site of the stimulator
electrode pads and possible damage to the stimulator.
-- Operation in close proximity (e.g. 1 m) to a shortwave or
microwave therapy ME equipment may produce instability in the
stimulator output.
--Application of electrode pads near the thorax may increase the risk of
cardiac fibrillation.
-- Stimulation should not be applied across or through the head, directly
on the eyes, over the mouth, on the front of the neck, (especially the
carotid sinus), or from electrode pads placed on the chest and the upper
back or crossing over the heart.
--Prevent inhalation or accidental swallowing of small parts
-- Prevent sharp parts from damaging the product.
-- Do not use accessories not specified by the manufacturer.