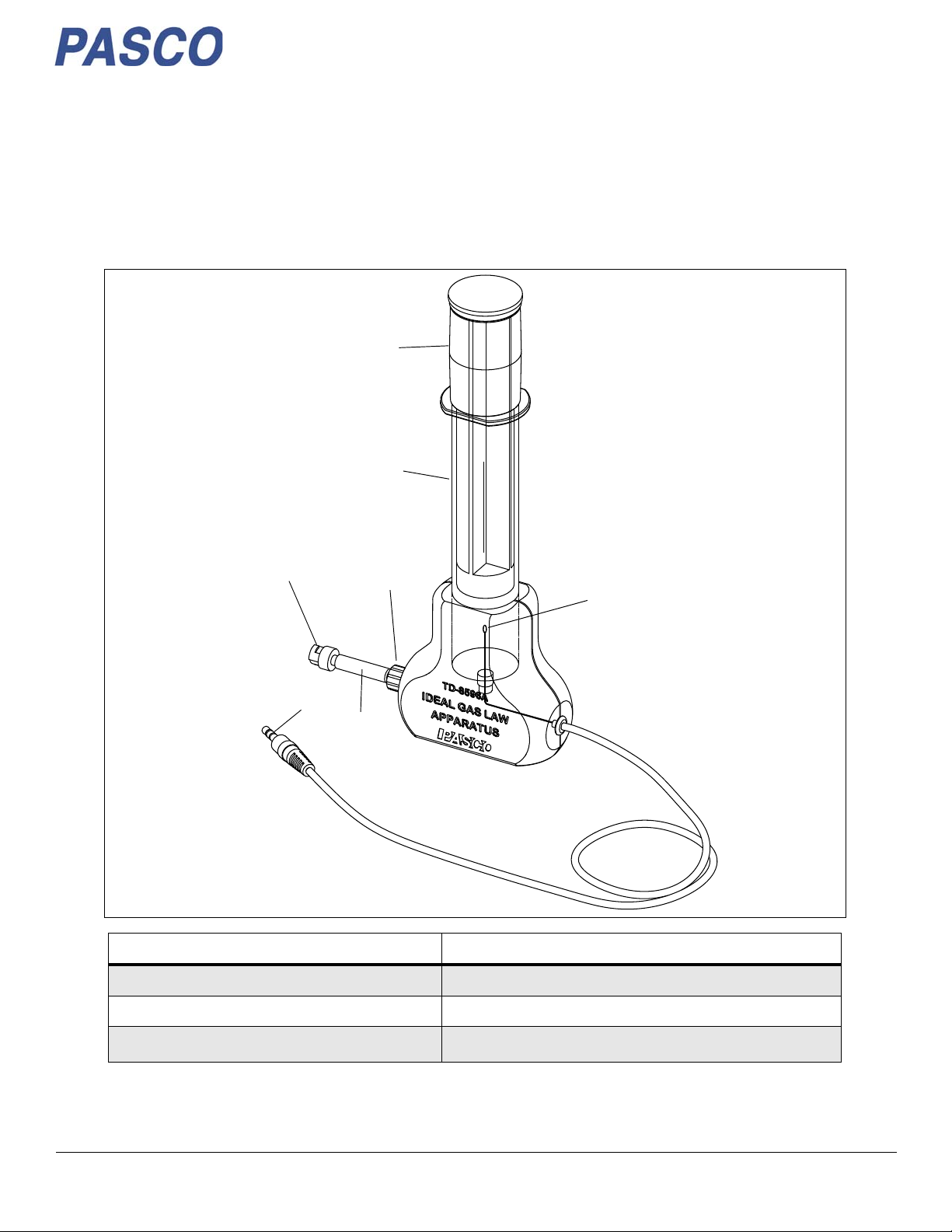
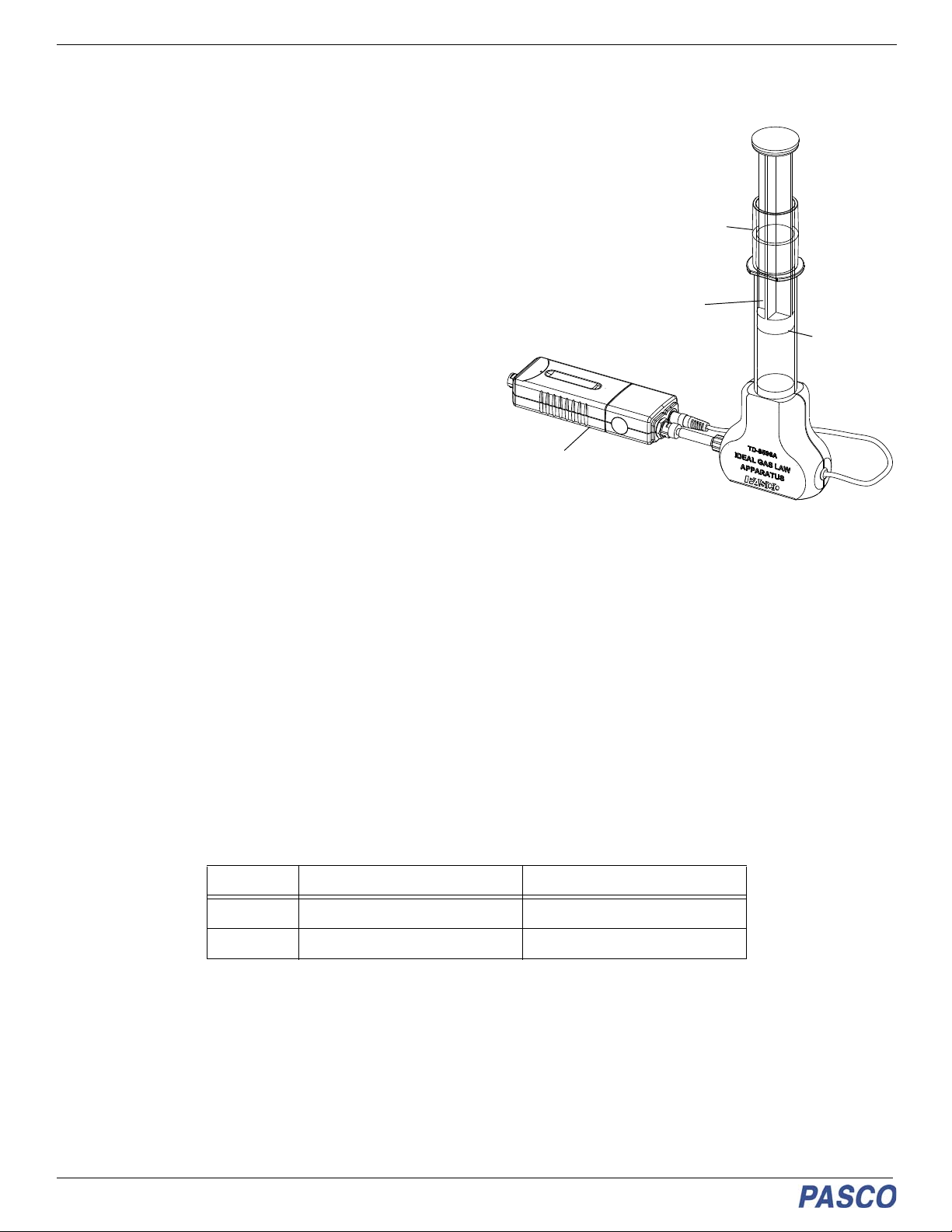
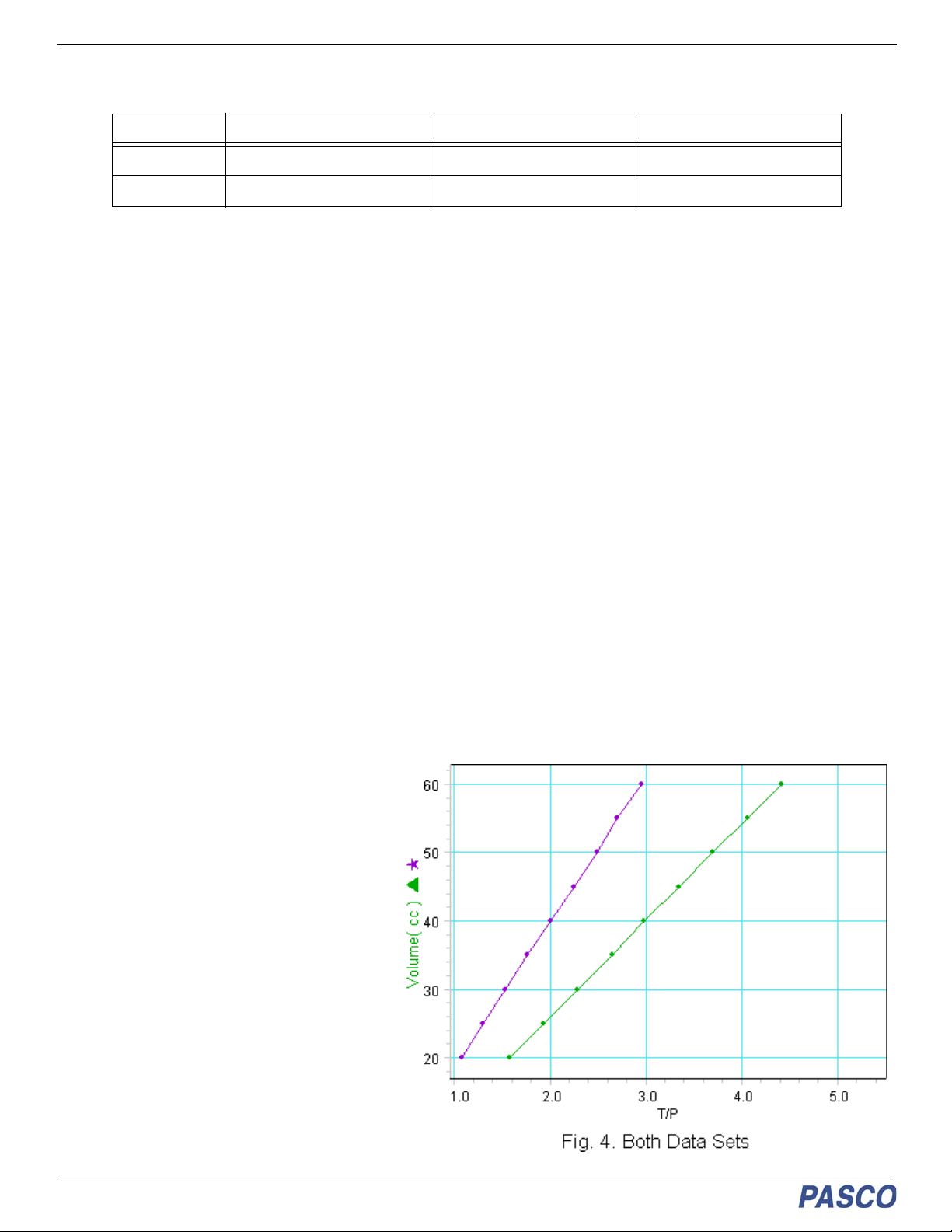
Ideal Gas Law Apparatus TD-8596A
6012-08420B
Questions
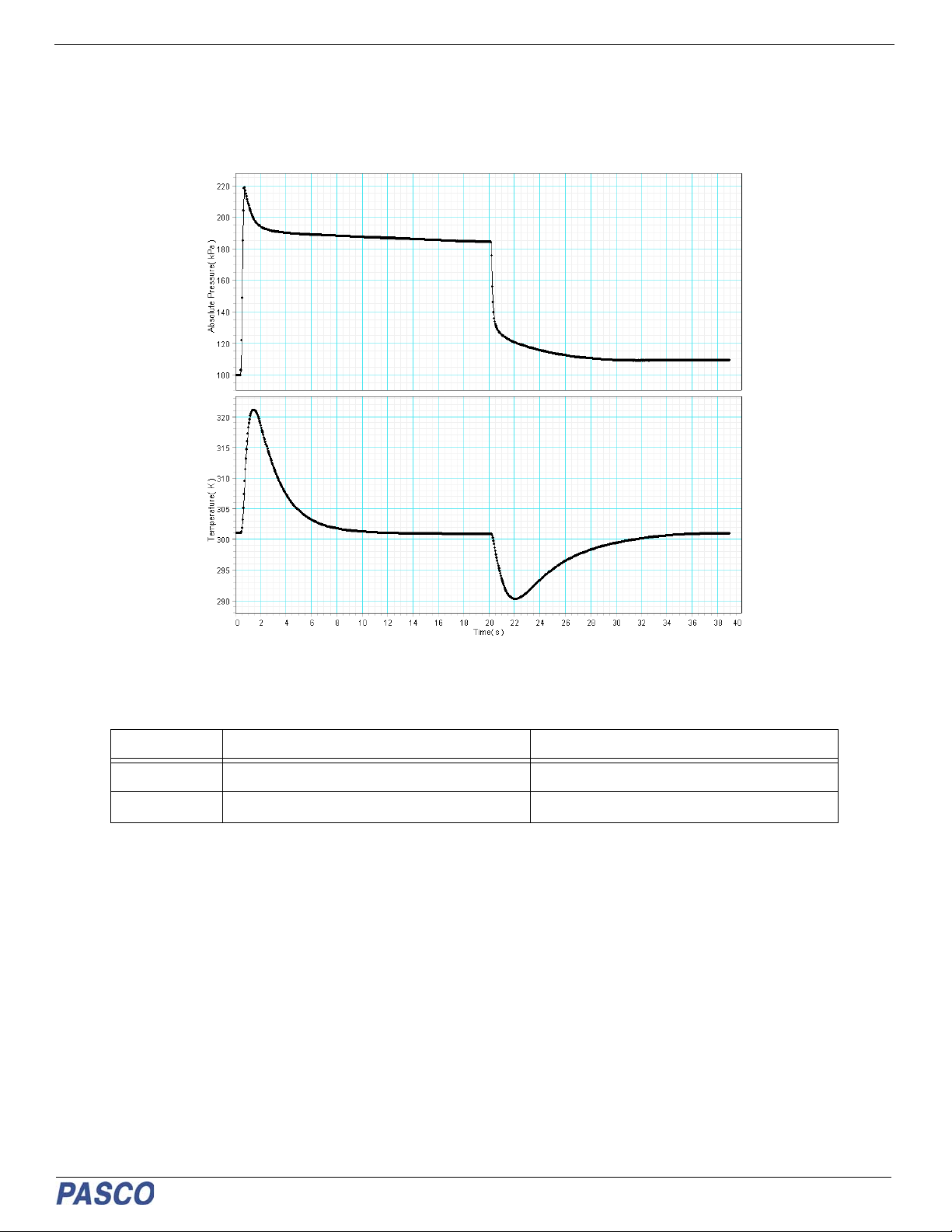
1. When the syringe volume is suddenly cut in half, the pressure changes by more than a factor of 2. Why does it momen-
tarily spike above 200 kPa?
2. When the syringe volume is suddenly cut in half, both the temperature and the pressure go up. After a short time, the
temperature approaches room temperature, but the pressure approaches some new, higher value. Why doesn't the
pressure decrease back to its original value like the temperature does?
3. When the plunger is released in the last part of the data run, what happens to the temperature? Why?
Safety
Read the instructions before using this product. Students should be supervised by their instructors. When using this
product, follow the instructions in this manual and all local safety guidelines that apply to you.
Technical Support
For assistance with any PASCO product, contact PASCO at:
Limited Warranty
For a description of the product warranty, see the PASCO catalog. For more information visit www.pasco.com/legal.
Copyright
This PASCO scientific Instruction Manual is copyrighted with all rights reserved. Permission is granted to non-profit educational institutions for
reproduction of any part of this manual, providing the reproductions are used only in their laboratories and classrooms, and are not sold for profit.
Reproduction under any other circumstances, without the written consent of PASCO scientific, is prohibited. Revised 07-16-18.)
Trademarks
PASCO, PASCO scientific, PASCO Capstone, PASPORT, and SPARKvue are trademarks or registered trademarks of PASCO scientific, in the United
States and/or in other countries. All other brands, products, or service names are or may be trademarks or service marks of, and are used to identify,
products or services of their respective owners. For more information visit www.pasco.com/legal.
FCC Statement
This Class A digital device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation.
CE Statement
This device has been tested and found to comply with the essential requirements and other relevant provisions of the applicable EU Directives.
Product End of Life Disposal Instructions:
This electronic product is subject to disposal and recycling regulations that vary by country and region. It is your responsibility to recycle your electronic
equipment per your local environmental laws and regulations to ensure that it will be recycled in a manner that protects human health and the
environment. To find out where you can drop off your waste equipment for recycling, please contact your local waste recycle/disposal service, or the place
where you purchased the product.
The European Union WEEE (Waste Electronic and Electrical Equipment) symbol (to the right) and on the product or its packaging
indicates that this product must not be disposed of in a standard waste container.
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Web: www.pasco.com
Phone: (916) 786-3800
(800) 772-8700