2
TABLE OF CONTENTS
Visual Index .................................................................................................................................................... 3
Section I - Familiarization ................................................................................................................................... 4
I. Safety Features ..................................................................................................................................... 4
A. DoorClampRing..................................................................................................................... 4
B. OpenDoorButton ................................................................................................................... 4
C. DoorHandle ............................................................................................................................ 4
D. RelayControl .......................................................................................................................... 4
II. Physical Characteristics ....................................................................................................................... 5
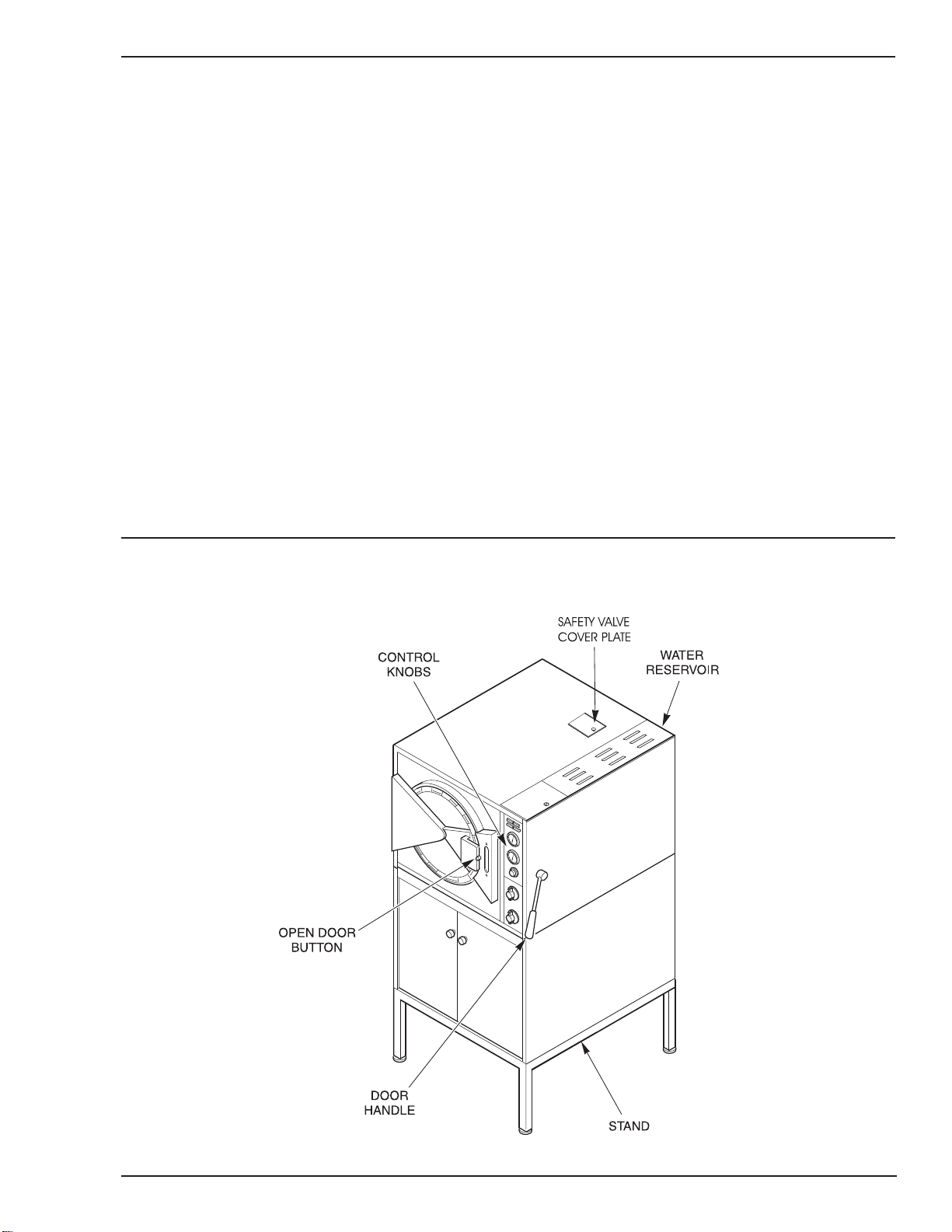
A. Exterior ................................................................................................................................... 5
B. Interior .................................................................................................................................... 5
C. InstallationRequirements........................................................................................................ 5
III.Electrical Requirements ....................................................................................................................... 6
IV.Controlsand Indicators......................................................................................................................... 6
A. Indicator Lights ....................................................................................................................... 6
B. Gauges ................................................................................................................................... 6
C. ControlKnobs ......................................................................................................................... 7
D. Audible Signal ......................................................................................................................... 7
E. WaterReservoir....................................................................................................................... 7
F. Stand....................................................................................................................................... 7
Section 2 - Preparation for Sterilization ............................................................................................................. 8
I.Handlingand Cleaning of Instruments ....................................................................................................
8
A. Handling..................................................................................................................................
8
B. Cleaning.................................................................................................................................. 9
II.Preparation Guide for CarbonSteelInstruments.................................................................................... 9
A. Handling.................................................................................................................................. 9
B. Cleaning.................................................................................................................................. 9
C. SterilizationPreparation .......................................................................................................... 9
D. InstrumentWrapping ...............................................................................................................
9
III.TrayPreparation and Loading ............................................................................................................... 9
A. GeneralGuidelinesforTray Preparation................................................................................... 9
B. UnwrappedTrays ................................................................................................................... 10
C. WrappedTrays and Instruments ............................................................................................ 11
D. Packs.................................................................................................................................... 12
IV.LiquidsPreparation............................................................................................................................. 13
Section 3 - Operation ......................................................................................................................................... 15
I.General ................................................................................................................................................ 15
II.OperatingProcedures for Normal Sterilization ..................................................................................... 15
A.UnlockDoor ........................................................................................................................... 15
B. Fill.......................................................................................................................................... 15
C.Load....................................................................................................................................... 15
D.LockDoor............................................................................................................................... 16
E.SetTime Control .................................................................................................................... 16
F.SetTemperature...................................................................................................................... 16
G.Vent ....................................................................................................................................... 16
H.UnlockDoor ........................................................................................................................... 16
I.Drying...................................................................................................................................... 16
III.Operating Procedures for LiquidsSterilization .................................................................................... 17
A. PreparationandSterilization .................................................................................................17
B. Vent ...................................................................................................................................... 17
C. CoolDown ............................................................................................................................ 17
D. Unlock Door .......................................................................................................................... 17