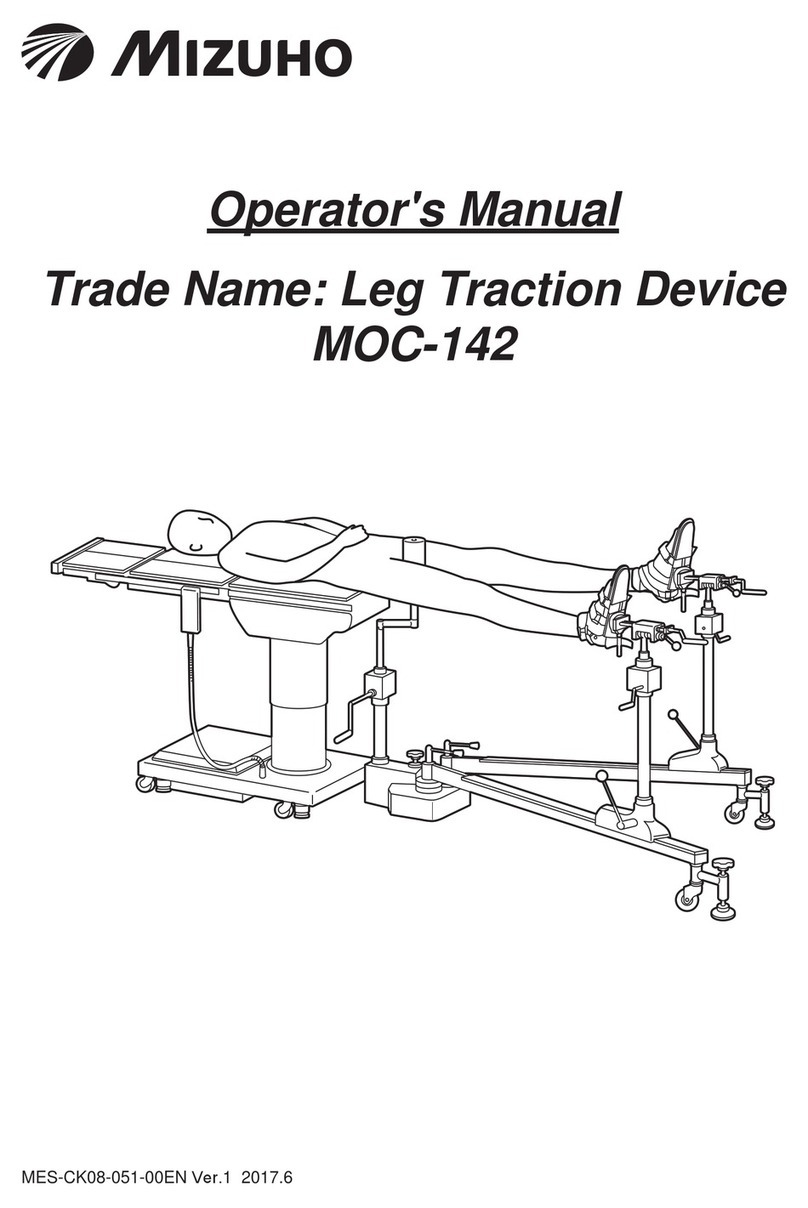
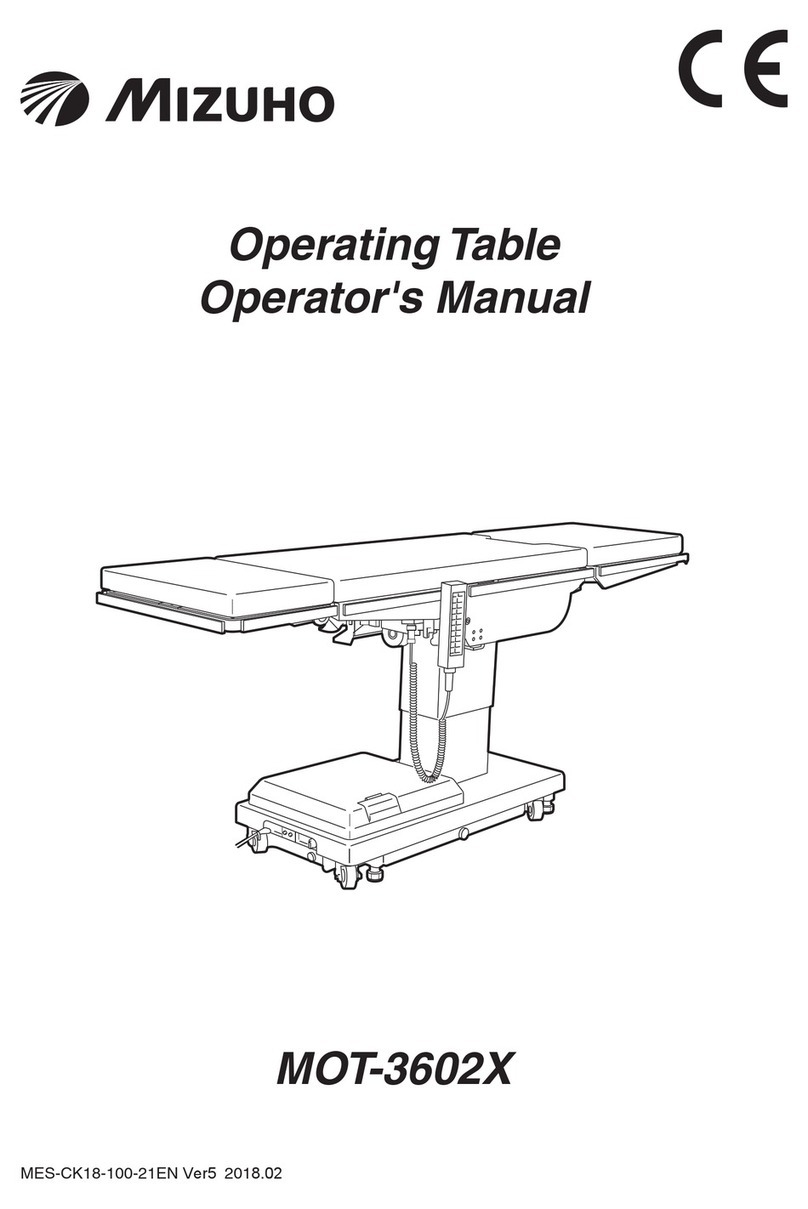
2/3
Adverse event
・Broken pieces of metal from the damaged instrument falling into
the patient.
Storage/Life
1. Please store the device in normal ambient temperature areas. Do not
store in areas of high humidity where the temperature may
dramatically vary causing condensation. Do not store on or near
chemicals as the chemicals may cause damage to the device.
2. Service life of this product: 1 years
(Subject to following manufacturer's specified maintenance,
inspection and proper storage requirements.)
Maintenance/Inspection
1. Check prior to each use
(1) Operational and functional checks
Conduct daily and pre-operation checks of this product to make
sure that it functions properly.
(2) Inspect the instrument for damage, fractures or cracks.
Examples of damage
2. Check after each use
(1) Immediately wash with clean water
(1)-1 If exposed to bleach or antiseptic solutions, immediately wash:
Wash and rinse with clean water immediately and immerse in
neutral enzyme detergent to remove any bleach or antiseptic
solution, which may contain chlorine or iodine and can damage
the instrument. Manually remove contaminated matter by
hand or with an ultrasonic-cleaner.
(1)-2 Further remove any remaining contamination with a plastic
brush.
(1)-3 Select a proper detergent for each decontamination method
and maintain appropriate density and handling.
(1)-4 Use a soft towel, a plastic brush or a water jet for cleaning
(1)-5 Avoid using metallic brushes or rough polishing agents,
applying excessive force, dropping or bumping the device, etc.
(1)-6 Reverse osmosis water is recommended to wash this product.
(1)-7 Only use reverse osmosis water for the final rinse.
※It is recommended to use a washer-disinfector for this device.
Thermal Disinfection can be used by following the manufacturer’s
defined parameters:
Thermal Disinfection Band: 90-93 °C/194.0-199.4°F, 5-10 minutes
(A0 value: 3000-12000) (reference EN ISO 15883-1)
(2) Fully dry this product immediately after washing it.
Do not leave it wet for a longer time than necessary as residual
water may damage the instrument.
(3) Use distilled water or reverse osmosis water
Use distilled water or reverse osmosis water to wash and sterilize
this product. Residual chlorine and organic matters in tap water
may cause stainings and/or rust and may damage the instrument
(4) Use a water-based anticorrosive lubricant
Lubricating oil is completely removed by washing. After washing
this product, apply a water-based, anticorrosive lubricant prior to
sterilization.
(5) Maintenance
(5)-1 After cleaning, visually inspect under ambient lighting and
confirm all dirt and debris has been removed. Confirm whether
or not the dirt in the inner lumen could be removed through the
attached mandolin.
(5)-2 If any dirt or debris is visible, repeat cleaning and lubrication
steps.
<Mizuho recommends the following procedures>
(1) Cleaning preparation
① When handling devices contaminated with blood or bodily fluids,
wear appropriate protective equipment such as masks, gloves,
eye protection, and waterproof aprons.
②Do not allow blood or bodily fluids on devices to dry.
③The instrument is fragile so handle carefully throughout the
cleaning and sterilization process so that the instrument and its
tips are not damaged.
(2) Cleaning
<Manual Procedure>
①Soak the product in cold tap water for 5 minutes, covering all
surfaces, and confirm that the lumen is filled with water.
② Rinse with tap water and clean with a plastic brush until all
visible residue is removed from the surface of the instrument.
Pass the attached mandolin through the inner lumen to remove
the dirt and rinse the inner lumen with cold tap water for 20
seconds using a water gun (hydrostatic pressure of 2 bars).
③Place the product into its washing basket and ultrasonically clean
at room temperature for 10 minutes in reverse osmosis water
with a neutral enzyme detergent dissolved to establish a 0.5%
detergent solution.
④Pass the attached mandolin through the inner lumen and rinse
with cold tap water for 20 seconds using a water gun
(hydrostatic pressure of 2 bars).
<Automated Procedure>
Connect the tool for washing of inner lumen of washer disinfector
with this product.
①Perform pre-cleaning for 3 minutes using tap water.
②Drain the water.
③Clean for 10 minutes in 50°C tap water with 0.3% alkaline
detergent solution.
④Drain the water.
⑤Rinse for 3 minutes with distilled or reverse osmosis water.
⑥Drain the water.
⑦Rinse for 2 minutes with distilled or deionized water for the final
rinse.
⑧Drain the water.
⑨Thermal Disinfection can be used by following the
manufacturer’s defined parameters: Thermal Disinfection Band:
90-93 °C/194.0-199.4°F, 5-10 minutes (A0 value:
3000-12000) (reference EN ISO 15883-1)
⑩Thoroughly dry prior to use or storage
(3) Only use a water-based, anticorrosive lubricant
Lubricating oil is completely removed by washing. Do not use
without lubricant oil to any sliding parts, or galling could occur. After
washing this product, apply a water-based anticorrosive lubricant
prior to sterilization.
3.Sterilization
The device must be sterilized in accordance with the manufacturer's
recommended sterilization procedures or the validated sterilization
conditions for which validity has been proven by medical
organizations in each country or region.
The recommended sterilization parameters are as follows,
sterilization
method
Pre-vacuum steam sterilization
(Autoclave sterilization)
sterilization
conditions
Sterilization temp. Retention time
132°C /270°F* 4min
134°C /273°F* 3min
*According to the Guideline for Disinfection and Sterilization in
Healthcare Facilities ,2008 (Update:May 2019)