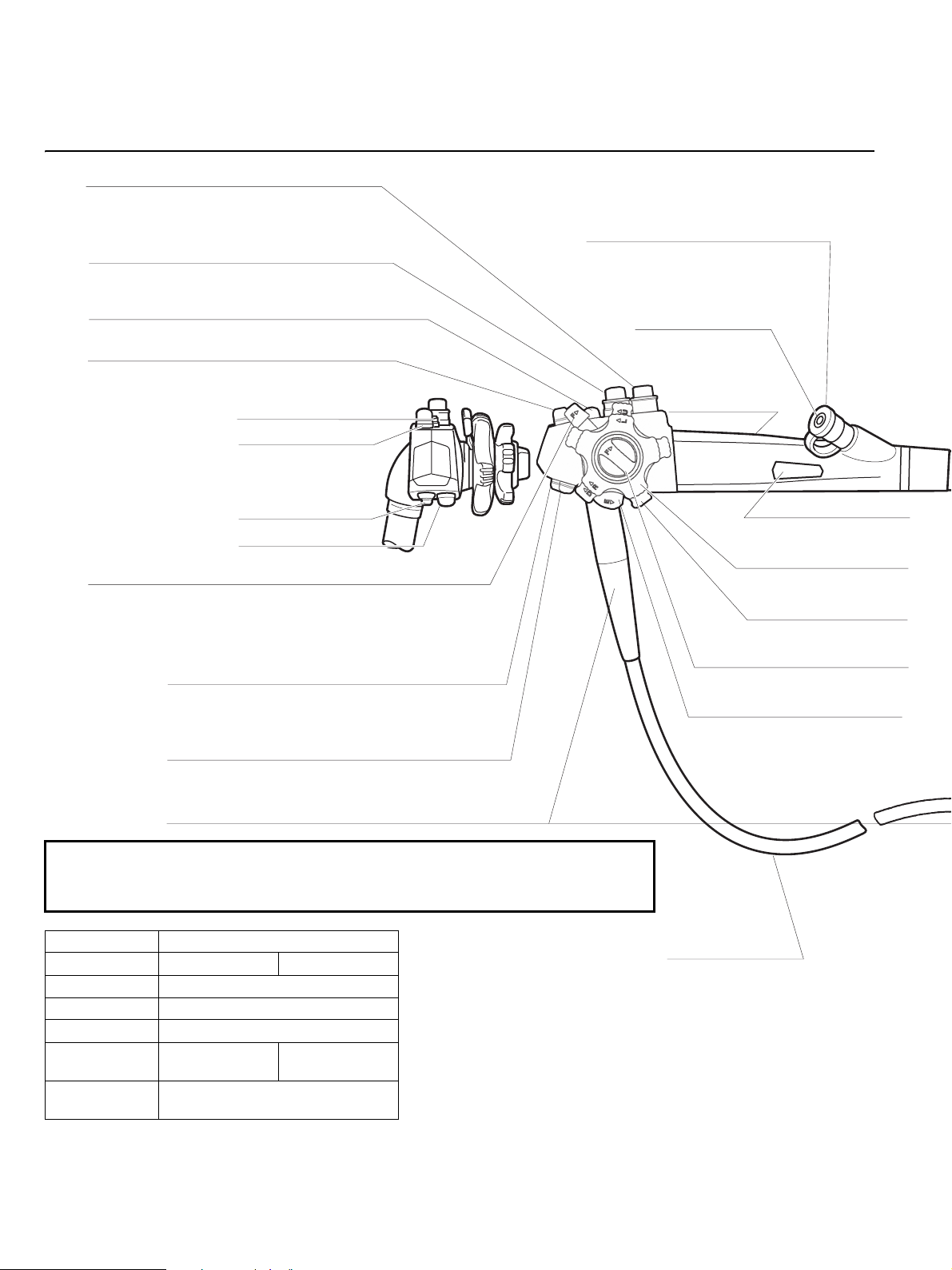
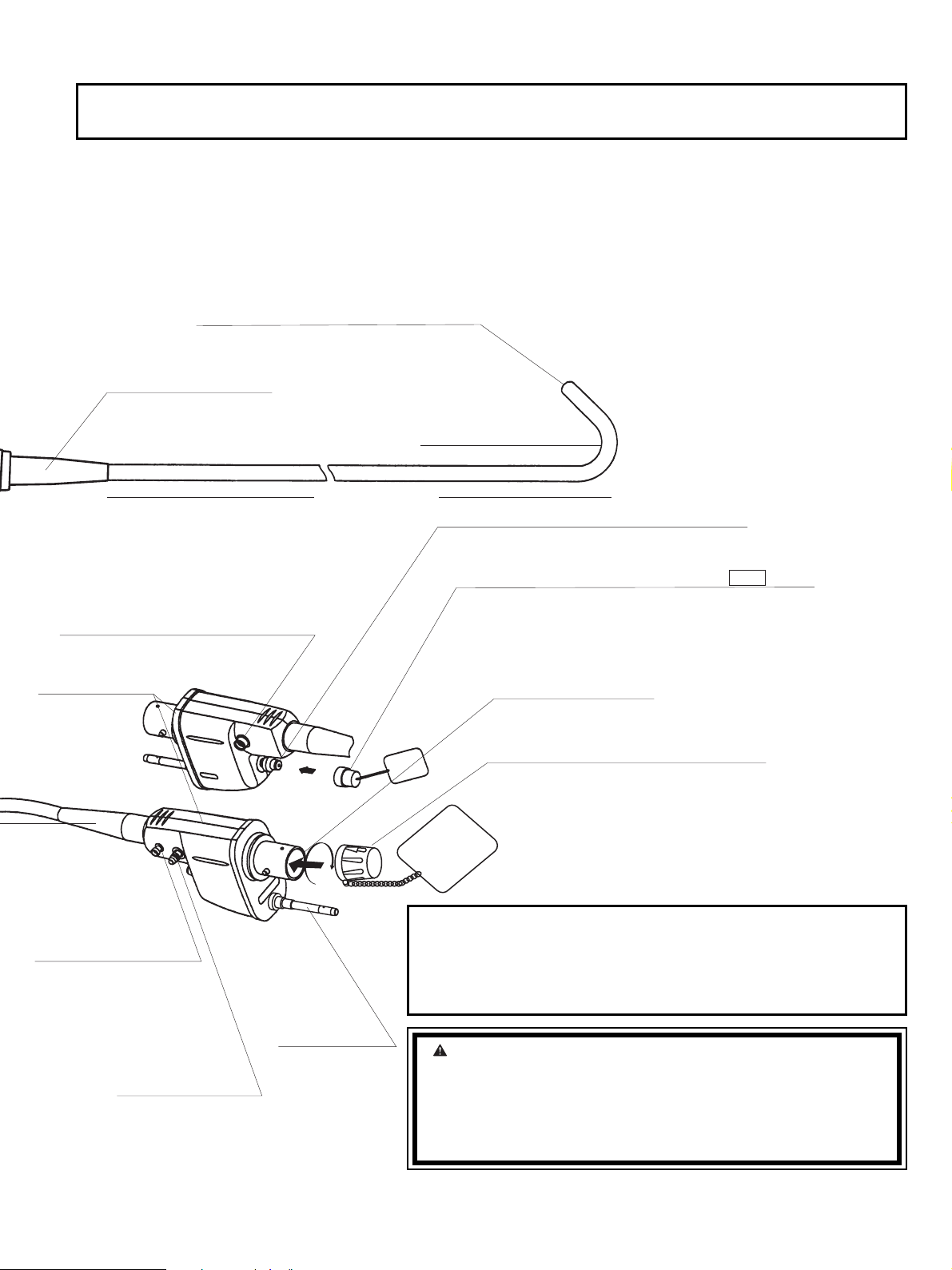
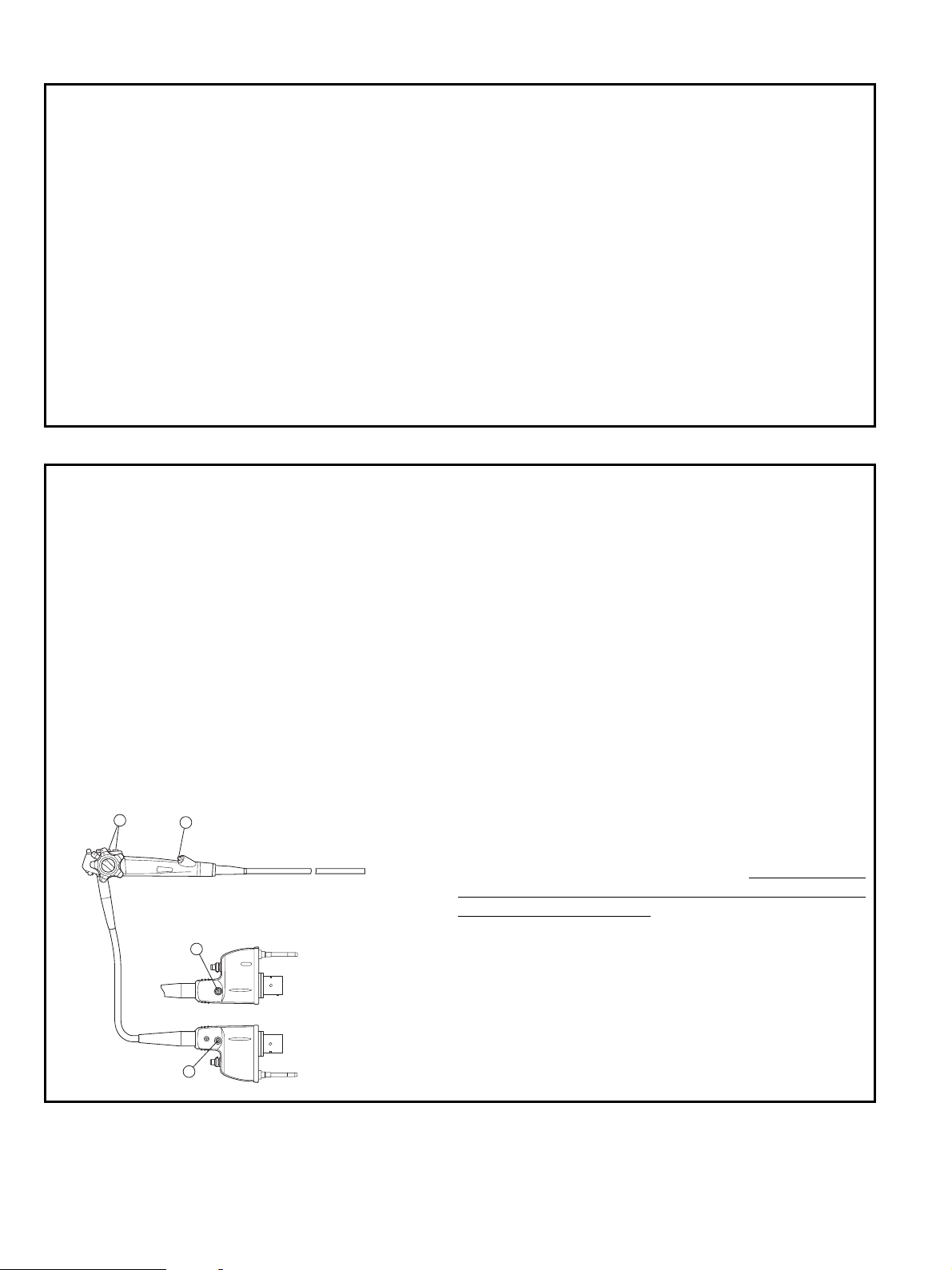
Intended Use / Indicationsfor use (Duodenoscopes)
The Video Duodenoscopes are intended to provide optical visualization of (via a video monitor), and therapeutic access to, Biliary Tract via
the Upper Gastrointestinal Tract. This anatomy includes, but is not restricted to, the organs, tissues, and subsystems: Esophagus, Stomach,
Duodenum, Common Bile, Hepatic and Cystic Ducts.
These instruments are introduced via the mouth when indications consistent with the need for the procedure are observed in adult and
pediatric patient populations.
Never use these endoscopes for any purpose other than that for which they have been designed.
The ED-3490TK can only be used with PENTAX Video Processors, Model EPK-i or EPK-1000.
Notes
Read this manual before operating, and save this book for future reference. Failure to read and thoroughly understand the information
presented in this manual, as well as those developed for ancillary endoscopic equipment and accessories, may result in serious injury
including infection by cross contamination to the patient and/or user. Furthermore, failure to follow the instructions in this manual or the
companion Instructions for Use (Reprocessing) may result in damage to, and/or malfunction of, the equipment.
It is the responsibility of each medical facility to ensure that only well educated and appropriately trained personnel, who are competent and
knowledgeable about the endoscopic equipment, antimicrobial agents/processes and hospital infection control protocol be involved in the
use and the reprocessing of these medical devices. Known risks and/or potential injuries associated with flexible endoscopic procedures
include, but are not limited to, the following: perforation, infection, hemorrhage, burns and electric shock.
This manual describes the recommended procedures for inspecting and preparing the equipment prior to its use.
It does not describe how an actual procedure is to be performed, nor does it attempt to teach the beginner the proper technique or any
medical aspects regarding the use of the equipment. For the cleaning and maintenance after its use, please refer to the separate
“Instructions for Use (Reprocessing)”.
The text contained in this manual is common for various types/models of PENTAX endoscopes and users must carefully follow only those
sections and instructions pertaining to the specific instrument models appearing on the front cover.
If you have any questions regarding any of the information in this manual or concerns pertaining to the safety and/or use of this equipment,
please contact your local PENTAX representative.
Sterility Statement
The instruments identified in this manual are reusable semi-critical medical devices. Since they are packaged non-sterile, they must be high-
level disinfected or sterilized BEFORE initial use. Prior to each subsequent procedure, they must be subjected to an appropriate cleaning
and either high-level disinfection or sterilization process.
Refer to the companion PENTAX Instructions for Use (Reprocessing) describing in detail the recommended instructions on the care,
cleaning, disinfection and sterilization of these endoscopes.
Conventions
Throughout this manual, the following conventions will be used to indicate a potentially hazardous situation which, if not avoided;
: could result in death or serious injury.
: may result in minor or moderate injury or property-damage.
: may result in property-damage. Also, advises owner/operator about important information on the use of this equipment.
Prescription Statement
Federal (U.S.A) law restricts this device to sale by or on the order of a physician or other appropriately licensed medical professional
The CE marking assures that this product complies with the requirements of the EC directive for safety.
Das CE Zeichen garantiert, daß dieses Produkt die in der EU erforderlichen Sicherheitsbestimmungen erfüllt.
Le logo CE certifie que ce produit est conforme aux normes de sécurité prévues par la Communauté Européenne.
II marchio CE assicura che questo prodotto è conforme alle direttive CE relative alla sicurezza.
La marca CE asegura que este producto cumple todas las directivas de seguridad de la CE.