Product Overview
These instruments photograph the subject of observation using a solid-state image sensor located at the endoscope tip under the
light transmitted from the processor/light source. The target of the observation is monitored by the physician using the endoscopic
image displayed on the video monitor. The endoscopic procedure is performed by inserting biopsy forceps and other endoscopic
accessories into the instrument channel inlet on the control body.
The bending section angulates in the intended direction and angle by operating the Angulation Control Lever; and air or fluids can
be suctioned from the distal end of the endoscope by operating the Suction Control Valve.
Indication for Use
The PENTAX Video Bronchoscopes (EB Family) have been designed to be used with a PENTAX Video Processor (including Light
Source), documentation equipment, video monitor, endo-therapy accessories (such as biopsy forceps) and other ancillary
equipment for endoscopy and endoscopic surgery within the airways and tracheobronchial tree.
Application
Medical purpose: Provide images for optical visualization, recording, and/or diagnostic aid.
Patient populations: Adults and lowercase pediatrics who have been determined by the physician to be appropriate candidates for
the use of this instrument.
Intended anatomical area: Airways and tracheobronchial tree
User: Medical doctors (expert approved by the medical safety officer to perform endoscopic examinations at each medical facility)
Place of Use: Medical facillity
Functions Used Frequently
The frequently used functions in these endoscope models are as follows:
•Angulation capability using control lever
•Remote control operation using remote buttons
•Suctioning function
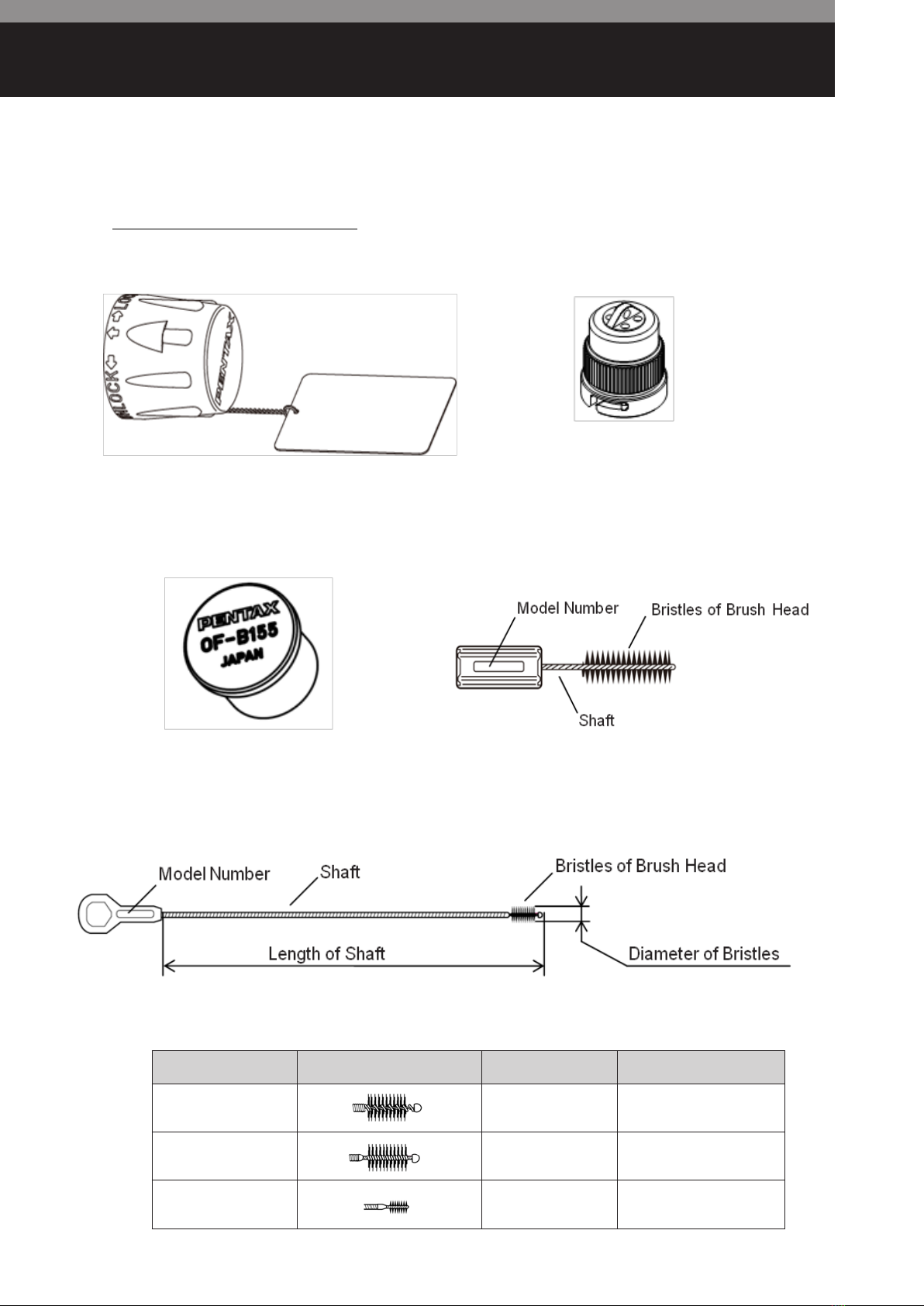
Removable Components
OF-B179 Suction Control Valve
OF-B190 Inlet Seal
Notes
Read this Instructions for Use (IFU) before reprocessing the endoscope, and save this book for future reference. Failure to read and
thoroughly understand the information presented in this IFU, as well as those developed for ancillary endoscopic equipment and
accessories, may result in serious injury, including infection by cross contamination to the patient and/or user. Furthermore, failure
to follow the instructions in this IFU or the companion Instructions for Use (operation) may result in damage to, and/ or malfunction
of, the equipment.
It is the responsibility of each medical facility to ensure that only well hyphen educated and appropriately trained personnel, who are
competent and knowledgeable about the endoscopic equipment, antimicrobial agents/processes, and hospital infection control
protocol be involved in the use and the reprocessing of these medical devices. Known risks and/or potential injuries associated with
flexible endoscopic procedures include, but are not limited to, the following: perforation, infection, hemorrhage, burns, and electric
shock.
This IFU describes the procedures for reprocessing and maintenance of the equipment after its use.
For inspection and preparation prior to its use, please refer to the separate Instructions for Use (Operation).
The text contained in this IFU is common to various types/models of PENTAX endoscopes, and users must carefully follow only
those sections and instructions pertaining to the specific instrument model in question.
If you have any questions regarding any of the information in this IFU or concerns pertaining to the safety and/or use of this
equipment, please contact your local PENTAX representative.