6
EverFlo / EverFlo QUser Manual
Warnings and Cautions
Warnings
A warning represents the possibility of harm to the operator or patient.
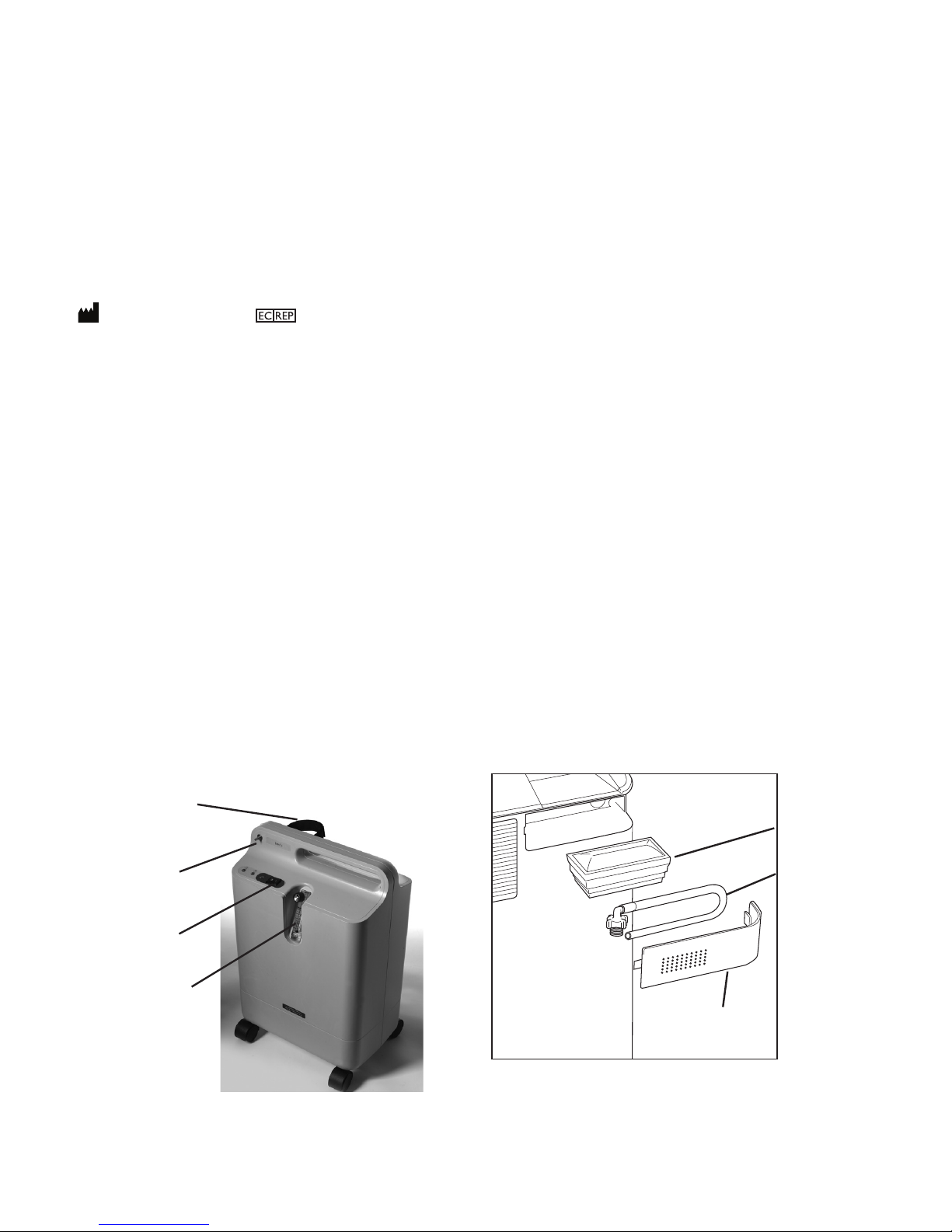
• For proper operation, your concentrator requires unobstructed ventilation. The ventilation ports are located
at the rear base of the device and at the side air inlet filter. Keep the device at least 15 to 30 cm away from
walls, furniture, and especially curtains that could impede adequate airflow to the device. Do not place the
concentrator in a small closed space (such as a closet). The device should not be used adjacent to or stacked
with other equipment. For more information, contact your home care provider.
• Do not remove the covers of this device. Servicing must be referred to an authorized and trained Philips
Respironics home care provider.
• In the event of an equipment alarm or if you are experiencing any signs of discomfort consult your home care
provider and/or your health care professional immediately.
• Oxygen generated by this concentrator is supplemental and should not be considered life supporting or life
sustaining. In certain circumstances oxygen therapy can be hazardous; any user should seek medical advice
prior to using this device.
• Where the prescribing health care professional has determined that an interruption in the supply of oxygen,
for any reason, may have serious consequences to the user, an alternate source of oxygen should be available
for immediate use.
• Oxygen vigorously accelerates combustion and should be kept away from heat or open flame. Not suitable for
use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide.
• Oxygen makes it easier for a fire to start and spread. Do not leave the nasal cannula or mask on bed coverings
or chair cushions, if the oxygen concentrator is turned on, but not in use; the oxygen will make the materials
flammable. Turn the oxygen concentrator off when not in use to prevent oxygen enrichment.
• Accessories used to connect the patient to the oxygen concentrator must be compatible to the requirements
of ISO 80601-2-69. Application accessories shall include a means to reduce the propagation of fire, including
having a fire stop device that stops fire and flow of oxygen to the patient.
• Do not smoke, allow others to smoke, or have open flames near the concentrator when it is in use. Smoking
during oxygen therapy is dangerous and is likely to result in facial burns or death.
• Do not use oil or grease on the concentrator or its components as these substances, when combined with
oxygen, can greatly increase the potential for a fire hazard and personal injury.
• Do not use the oxygen concentrator if either the plug or power cord is damaged. Do not use extension cords
or electrical adapters.
• Do not attempt to clean the concentrator while it is plugged into an electrical outlet.
• Device operation above or outside of the voltage, LPM, temperature, humidity and/or altitude values specified
may decrease oxygen concentration levels.
• Your home care provider is responsible for performing appropriate preventive maintenance at the intervals
recommended by the device manufacturer.
• Portable and mobile RF communications equipment can affect Medical Electrical Equipment. See the EMC
section of this manual for distances to observe between RF communications equipment and the device to
avoid interference.
• Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed and put into
service according to the EMC information provided in this manual.