Version of Tuesday, 27 November 2018 / V2.0
Table of contents
1. General Information................................................................................................... 3
About this manual .......................................................................................................................................... 3
Contact ........................................................................................................................................................... 3
Symbols and Marks ........................................................................................................................................ 4
2. Device presentation ................................................................................................... 6
Interfaces........................................................................................................................................................ 7
Indications ...................................................................................................................................................... 8
Contraindications ........................................................................................................................................... 8
Precautions..................................................................................................................................................... 8
Functions ........................................................................................................................................................ 9
Warnings ...................................................................................................................................................... 10
3. Device Installation ................................................................................................... 12
Minimum configuration requirements......................................................................................................... 12
Installation.................................................................................................................................................... 13
Starting a measurement............................................................................................................................... 13
4. Identification and update of software ...................................................................... 14
Identification of the embedded software version........................................................................................ 14
Identification of the PhysioFlow® software V2 ............................................................................................ 14
Software update........................................................................................................................................... 14
5. Maintenance, Transport, Storage and Disposal ........................................................ 15
Maintenance ................................................................................................................................................ 15
Hardware ................................................................................................................................................. 15
Software................................................................................................................................................... 15
Storage and transport .................................................................................................................................. 16
Disposal ........................................................................................................................................................ 16
Appendix A: Accessories.................................................................................................. 17
Appendix B: Specifications .............................................................................................. 18
Environmental .............................................................................................................................................. 18
Electric and mechanical................................................................................................................................ 18
Electromagnetic compliance ........................................................................................................................ 19
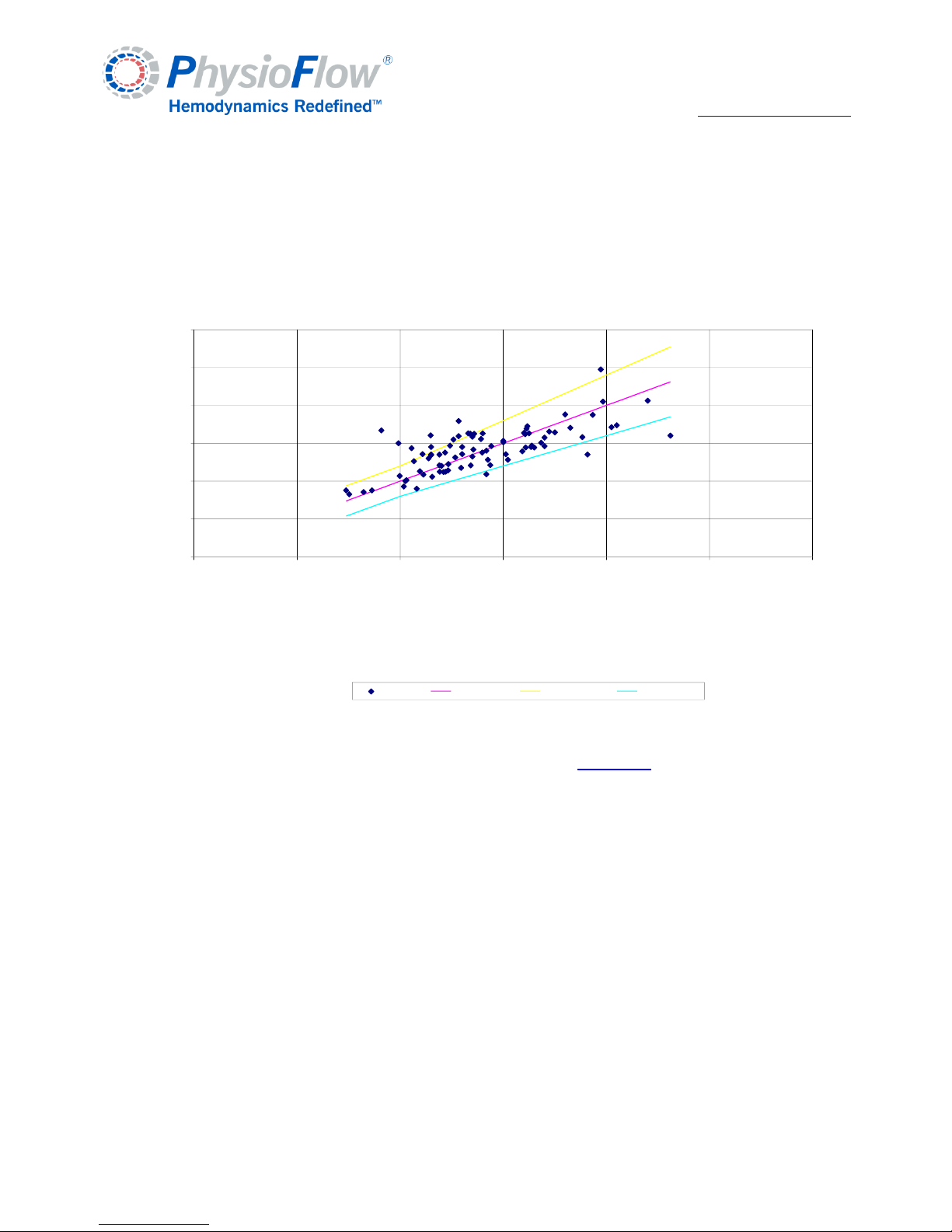
Appendix C: Physiological parameters............................................................................. 22