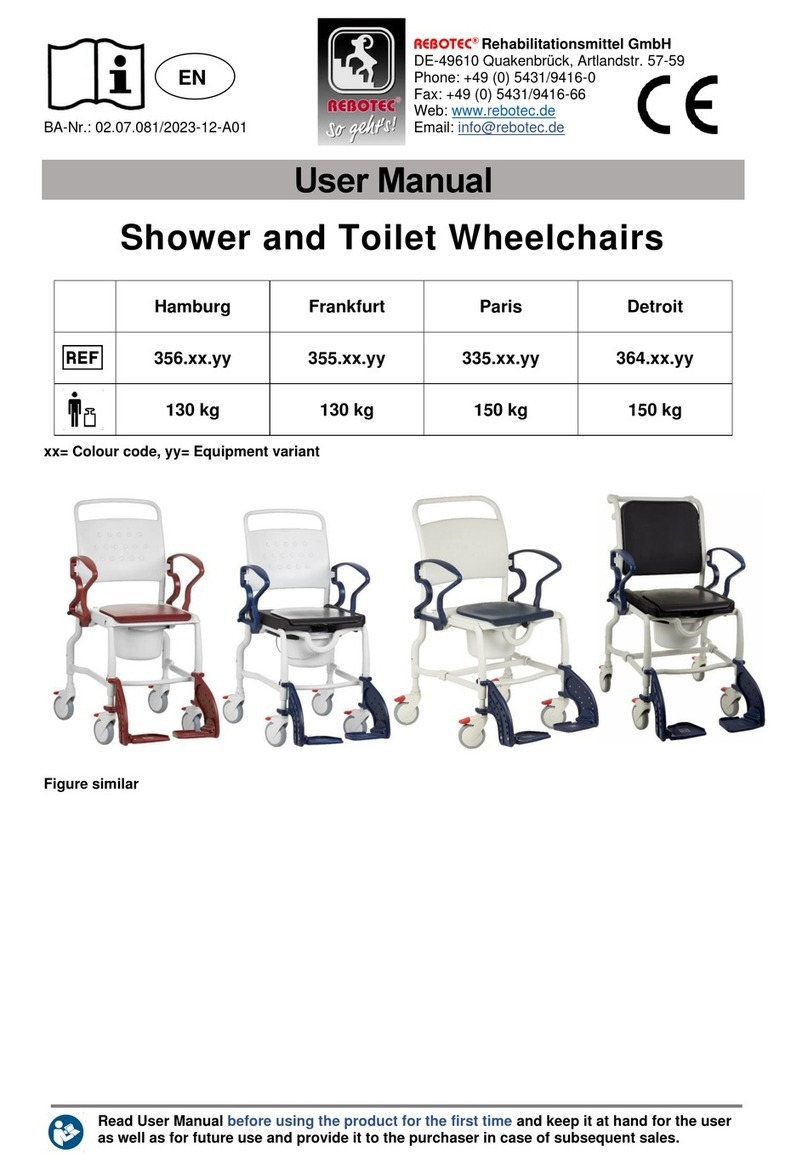
•Sitting down, getting up and transferring of a patient
should be done with the help of a trained caregiver,
depending on the patient's state of health.
•Patients who are prone to uncontrolled actions or
movements must be supervised when using the
chair.
•The chair must only be moved on a slope or incline
with the help of a caregiver. Generally, on an uphill
slope the chair should be pushed forward and on a
downhill slope it should be pulled backward.
Caution: Danger of slipping!
•Before use, check that the seat surface is firmly
clamped in the seat frame.
•Always lock the parking brakes before using the
chair to prevent the chair from rolling away.
•When sitting down, do not sit on the front edge of
the seat or on the seat cushion; the user should
take up the entire seat surface.
Caution: Danger of tipping over!
•The chair must only be used with the armrests
locked in place when sitting and moving about.
•The chair must only be pushed by the push handle.
•The footrests must be swivelled down or folded up
before sitting down/standing up. Do not stand on
the footrests!
•Do not move the chair against door thresholds,
edges or other uneven surfaces.
•Only use the product indoors and on flat, skid-proof
surfaces.
•Only move the product at a moderate pace.
•Never lean out of the chair. If the swivel castors are
in an unfavourable position (pointing inwards), the
chair’s stability is limited.
Caution: Danger of breakage!
•The product is only suitable for the intended use.
Note the maximum body weight allowed. See 2.
•Never carry or lift the product while a patient is sit-
ting on it.
•Do not pull or hold on to additionally attached com-
ponents or accessories.
Caution: Danger of pinching or catching!
•Do not leave the patient unattended during use.
•When folding the armrests up or down, do not reach
into the joint guide to avoid any trapping of body
parts etc. in them. Do not put any body parts into
the armrests.
•When folding down the footrests, take care to avoid
trapping any body parts. Do not insert any body
parts into the cut-out in the heel area.
•When pulling out and pushing in the toilet bucket,
there is a risk of soft parts of the patient being
trapped. To prevent injuries, the bucket may only
be pulled out or pushed in when no user is sitting in
the chair.
Caution: Risk of injury!
•There is a risk of injury with surface temperatures >
40 °C. Therefore, do not expose the product to high
temperatures (e.g. sunlight, radiators, hot water).
Allow the product to cool down before use.
If the product is used by patients with insen-
sitive skin (no temperature perception or skin
damage), a heat check (e.g. touching with
the back of the hand) must be carried out by the nurs-
ing staff.
5 Incident reporting
•Serious incidents in connection with the product
must be reported immediately to the manufacturer
and Federal Institute for Drugs and Medical De-
vices (BfArM).
▪Use the reporting functions and provided
forms.
Manufacturer: pms@rebotec.de
6 Warranty
•The manufacturer grants a warranty of 12 months
for this product. The prerequisites are the general
terms and conditions (www.rebotec.de/agbs), as
well as the intended use. The statutory warranty
provisions shall apply.
•Unauthorised modifications to this product will void
the product’s conformity and the warranty.
6.1 Complaints
•Please contact us before returning the product.
•To reduce transport damage, use the original pack-
aging if possible.
•The Infection Protection Act must be complied with.
•Please make sure that the product does not pose a
risk of infection when returned.
•Enclose the supplied information sheet on safety
with the product.
•Costs may be incurred for returns that are not
marked as harmless.