6 7
I. General Information
Important notes on the operation of the reading magnifier MANOtouch4.
Please read them carefully prior to the operation of the reading magnifier!
Notes on the electromagnetic compatibility (EMC)
This reading system is suited for use in all set-ups including the domicile
that are connected directly to a public power supply for buildings with
residential purposes.
This reading system is designed for the use in the indicated electro-
magnetic environment. The user should ensure the use in the indicated
environment.
This reading system operates on HF-Energy exclusively for its own func-
tion. Therefore the emitted interference of high frequency disturbances
is very low and the interference with other appliances operated close-by
is improbable. When operating the reading system simultaneously with
other systems, however, the possibility of disturbances of the system or
other systems should not be neglected.
For this reason it should be ensured that the system is not placed directly
beside or on top of another system. If the set-up of this system in the
proximity of medical, measuring devices cannot be avoided,the operator
of these devices has to be informed that it is necessary to observe the
performance of the system to control the conventional use of the system
in the chosen set-up.
Electronic devices are sensitive towards electric discharge and towards
high frequency electromagnetic fields.
Attention:
In order to avoid high frequency disturbances the operator should
respect a minimum distance of one meter to portable and mobile
HF-telecommunication devices.
The device may not be used in the patients environment.
Storage of the user manual
The user manual is an essential part of MANOtouch4. Please keep
the manual in a safe place for future reference.
Product description
MANOtouch4 is an electronic magnifier for text and pictures for perma-
nent and private use.
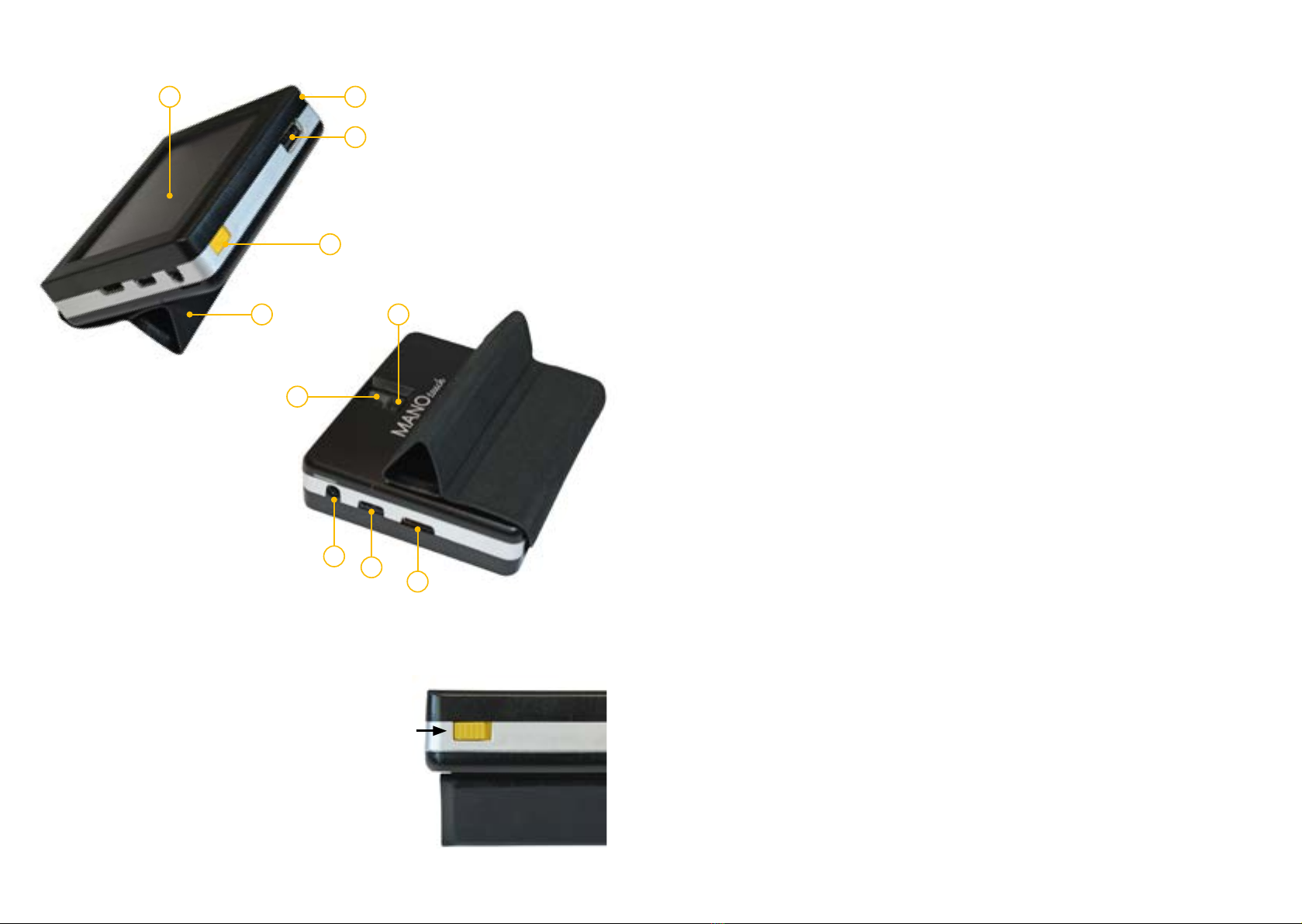
• clear, virtual touch display (approx. 9 cm in diagonal)
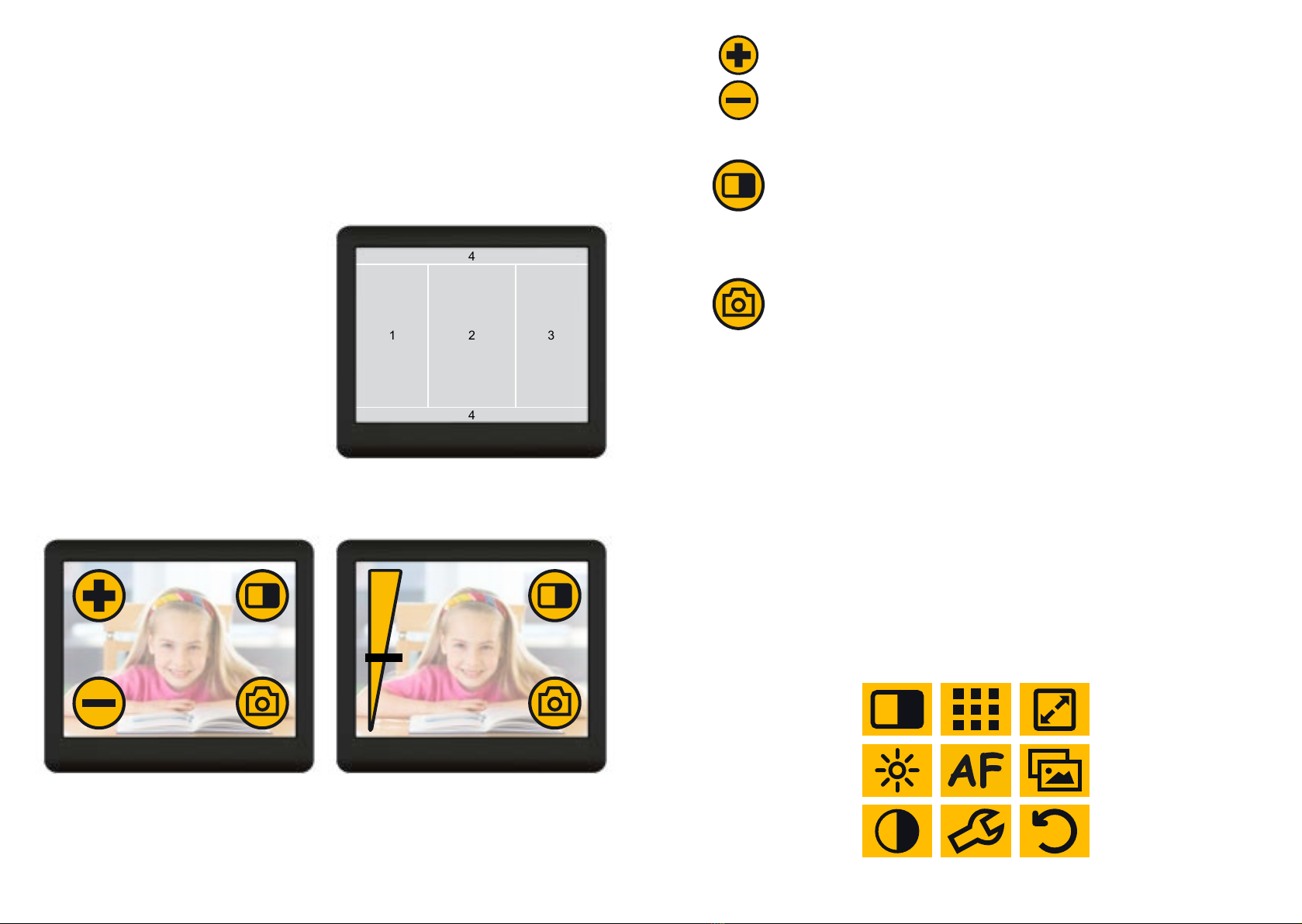
• easy, intuitive operation via virtual, customisable buttons
with optional tactile feed back (sensitively or acoustically)
• high resolution picture quality, contrast enhanced and flicker
free
• continuous magnification from approx. 1x to 20x, thanks to
cutting-edge camera technology
• multifunctional protection cover which can be also used as
comfortable reading/writing stand
• distance view mode, trapezoid balance, time display, HDMI
port, auto ON/OFF, auto focus ON/OFF, inductive charger
(available as an option)
• due to its light weight and its comfortable design,
MANOtouch4 can be comfortably used at home or “on the
go”.
Medical Device
MANOtouch4 is classified as a medical device, class I according to
(EU) 2017/745, Annexe VIII, standard 13. For the distribution to countries
outside the EU the respective country-specific guidelines have to be
regarded.
Purpose
This electronic magnifier is a medical device to assist with the magnifica-
tion of printed text and pictures. Visually impaired people can compensate
for their loss of visual acuity by using this device. For private appliance
only.