5
TABLE OF CONTENTS
1. GENERAL INFORMATION........................................................................................................ 8
1.1. DEAR COSTUMER................................................................................................................................ 8
1.2. INDICATONS FOR USE ........................................................................................................................ 8
1.3. CONTRAINDICATION............................................................................................................................ 8
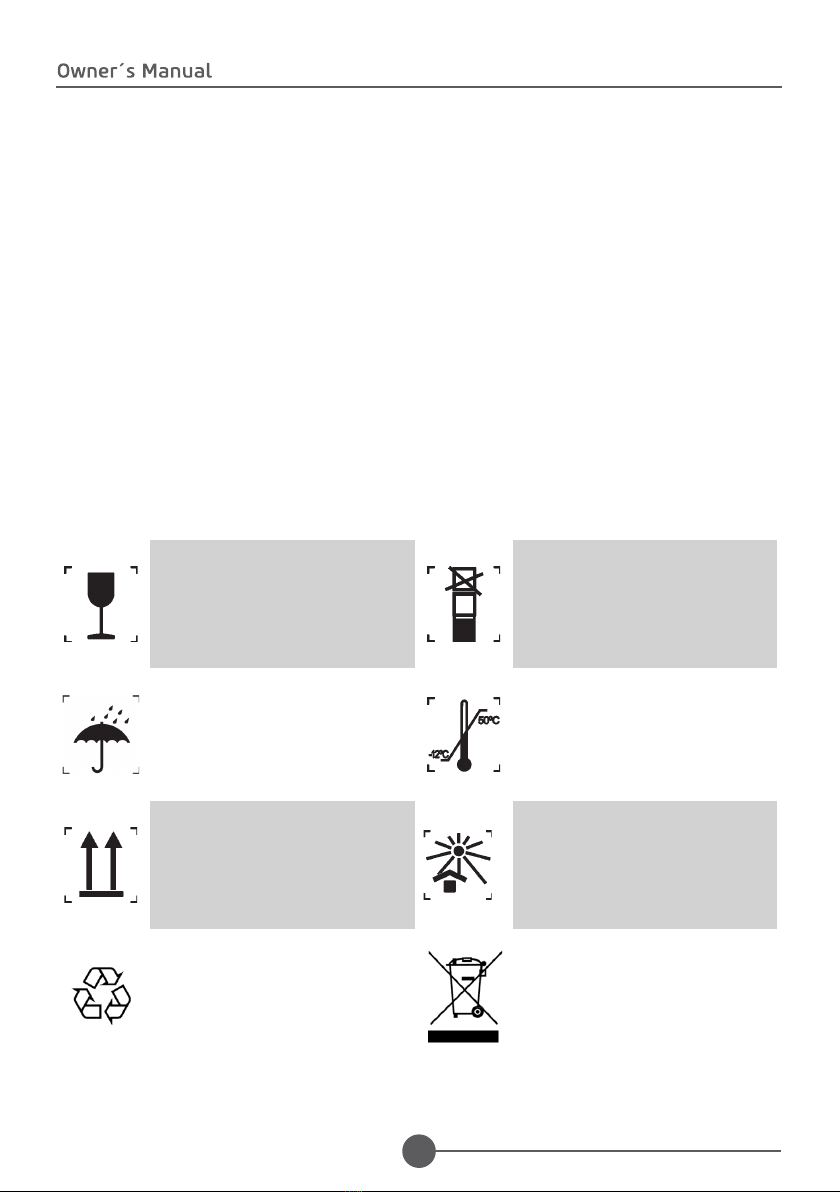
1.4. SYMBOLOGY........................................................................................................................................ 8
2. WARNINGS, PRECAUTIONS AND RECOMMENDATIONS ........................................................ 11
3. SYSTEM GENERAL DESCRIPTION .......................................................................................... 16
3.1. SYSTEM DESCRIPTION....................................................................................................................... 16
3.2. APPLICATION SPECIFICATION........................................................................................................... 16
3.2.1. Operation Principles ........................................................................................................................ 16
3.2.2. Signicant physical characteristics.............................................................................................. 16
3.2.3. User’s prole ..................................................................................................................................... 17
3.3. MAIN PRODUCT COMPONENTS........................................................................................................ 18
3.3.1. Light.................................................................................................................................................... 18
3.3.2 Arm ...................................................................................................................................................... 19
3.4. APPLIED PARTS.................................................................................................................................. 20
3.5. LABEL POSITIONING.......................................................................................................................... 20
3.6 SYSTEM REQUIREMENTS .................................................................................................................. 20
3.6.1. Place of Installation......................................................................................................................... 20
4. OPERATION ............................................................................................................................ 22
4.1 INITIAL PREPARATION....................................................................................................................... 22
4.2. ACTIVATING THE LIGHT ..................................................................................................................... 22
4.3 MOVEMENT OF THE HEADSTOCK .................................................................................................... 24
5. CLEANING AND DISINFECTION.............................................................................................. 26
6. PROBLEMS DIAGNOSTIC........................................................................................................ 28
6.1. PROBLEMS SOLUTION....................................................................................................................... 28
7. INSPECTION AND MAINTENANCE ......................................................................................... 30
7.1. PERIODIC INSPECTION ...................................................................................................................... 30
7.2. PREVENTIVE MAINTENANCE ............................................................................................................ 30
7.3. CORRECTIVE MAINTENANCE............................................................................................................. 31
7.4. ALLIAGE AUTHORIZED SERVICE NETWORK ................................................................................... 32
8. WARRANTY ............................................................................................................................ 34
8.1. WARRANTY TERMS............................................................................................................................ 34
8.2. WARRANTY PERIOD........................................................................................................................... 34
9. RULES AND REGULATIONS.................................................................................................... 36
10. TECHNICAL SPECIFICATIONS ................................................................................................. 38
10.1. EQUIPMENT CLASSIFICATION........................................................................................................... 38
10.2. DEVICE INFORMATION....................................................................................................................... 38
10.3. ENVIRONMENTAL CONDITIONS........................................................................................................ 39
10.4. LIGHT BEAM PROJECTION ................................................................................................................ 39
10.5 DENTAL LIGHT DIMENSIONS ............................................................................................................ 40