978-922-1832 ●www.sagescience.com ●support@sagescience.com
BluePippin Operations Manual 460013 Rev F iv
10 PREPARING A CASSETTE...........................................................................................10-1
10.1 Visually Inspect the Cassette ......................................................................................10-1
10.2 Prepare the Cassette for Loading ................................................................................10-3
10.3 Continuity Test...............................................................................................................10-4
11 LOADING SAMPLES.....................................................................................................11-1
12 RUNNING A PROTOCOL..............................................................................................12-1
12.1 Overview .........................................................................................................................12-1
12.2 Starting a Run.................................................................................................................12-2
12.3 Monitoring a Run ...........................................................................................................12-3
12.4 Log Files .........................................................................................................................12-5
13 SAMPLE COLLECTION ................................................................................................13-1
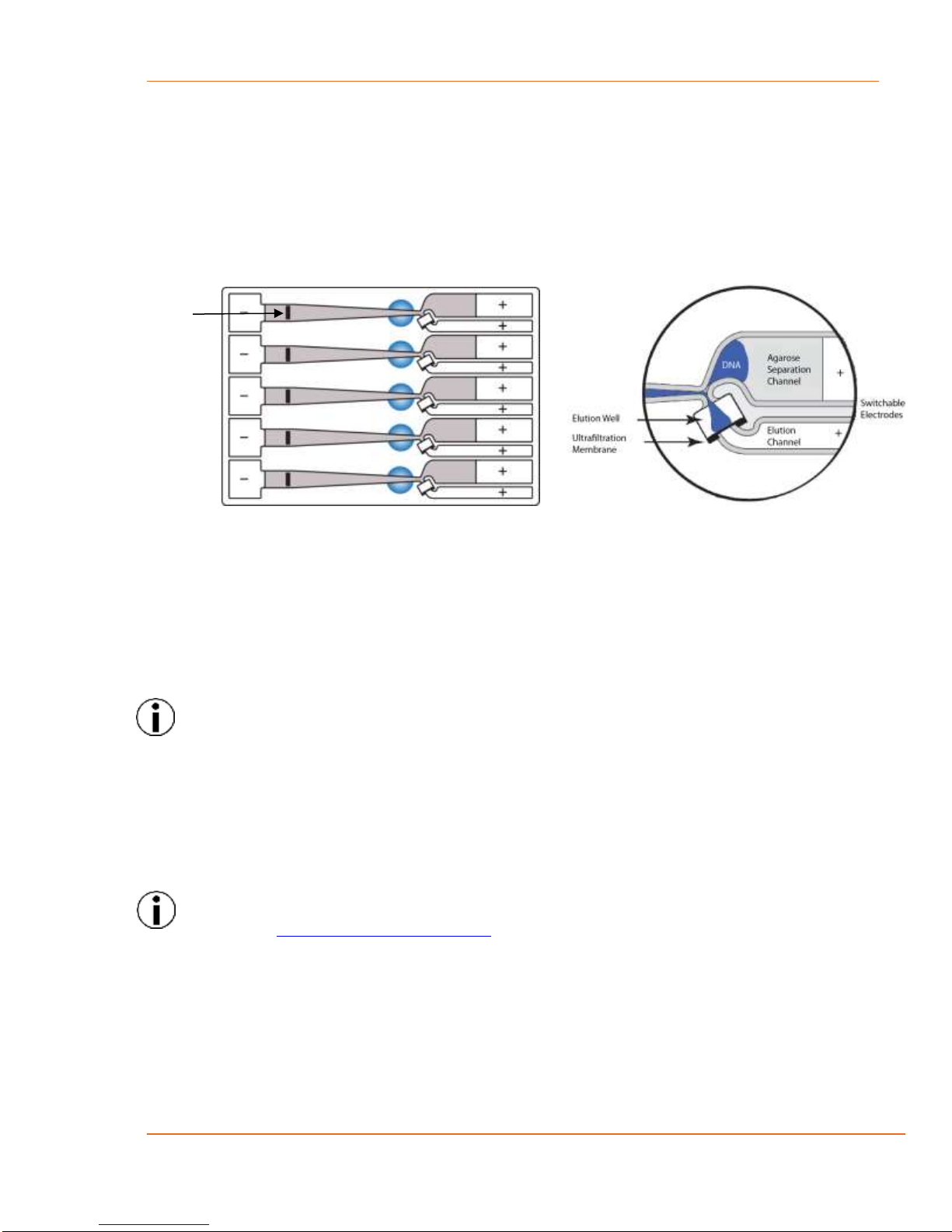
13.1 Overview .........................................................................................................................13-1
14 SAMPLE RECOVERY AND YIELD................................................................................14-1
14.1 Overview .........................................................................................................................14-1
14.2 Intrinsic Sample Recovery on the BluePippin .............................................................14-1
14.3 Improving Product Yield by Selecting a Wider Size Range........................................14-2
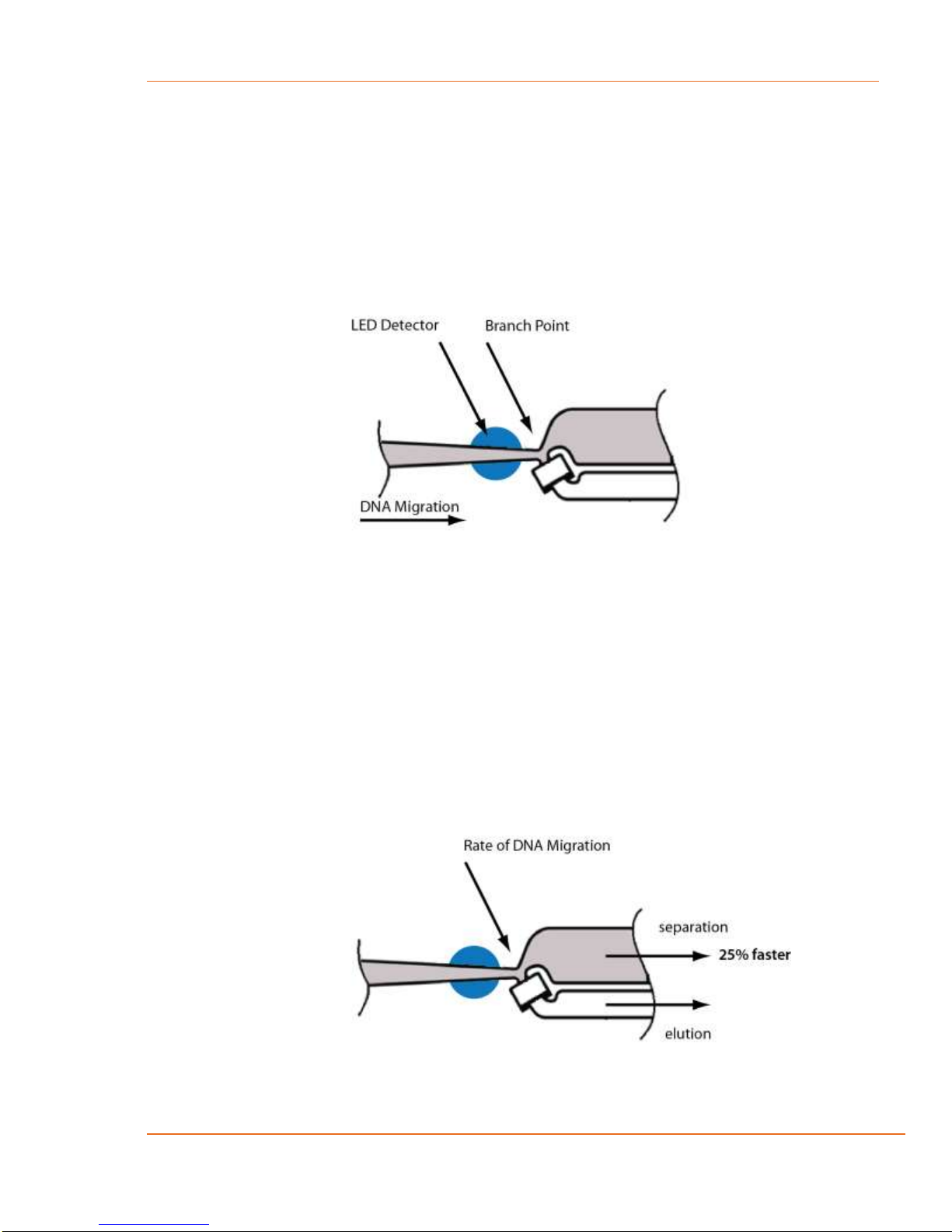
14.4 Improving Product Recovery with the Field Reversal .................................................14-3
14.5 HMW DNA: Recommendations to Improve Yield..........................................................14-4
15 SYSTEM VALIDATION..................................................................................................15-1
16 SYSTEM OPTIONS TAB ...............................................................................................16-1
17 RUNNING IN MANUAL MODE......................................................................................17-1
18 ANALYZING RUNS - LOG REVIEW TAB......................................................................18-1
19 MANAGING FILES -- FILE MANAGER TAB.................................................................19-1
19.1 Overview ..........................................................................................................................19-1
19.2 File Types.........................................................................................................................19-2
19.3 Transferring files.............................................................................................................19-3
20 UPGRADING SOFTWARE ............................................................................................20-1
20.1 Extracting the Files to a USB flash drive .....................................................................20-1
20.2 Upgrading the Pippin Instrument Software..................................................................20-3