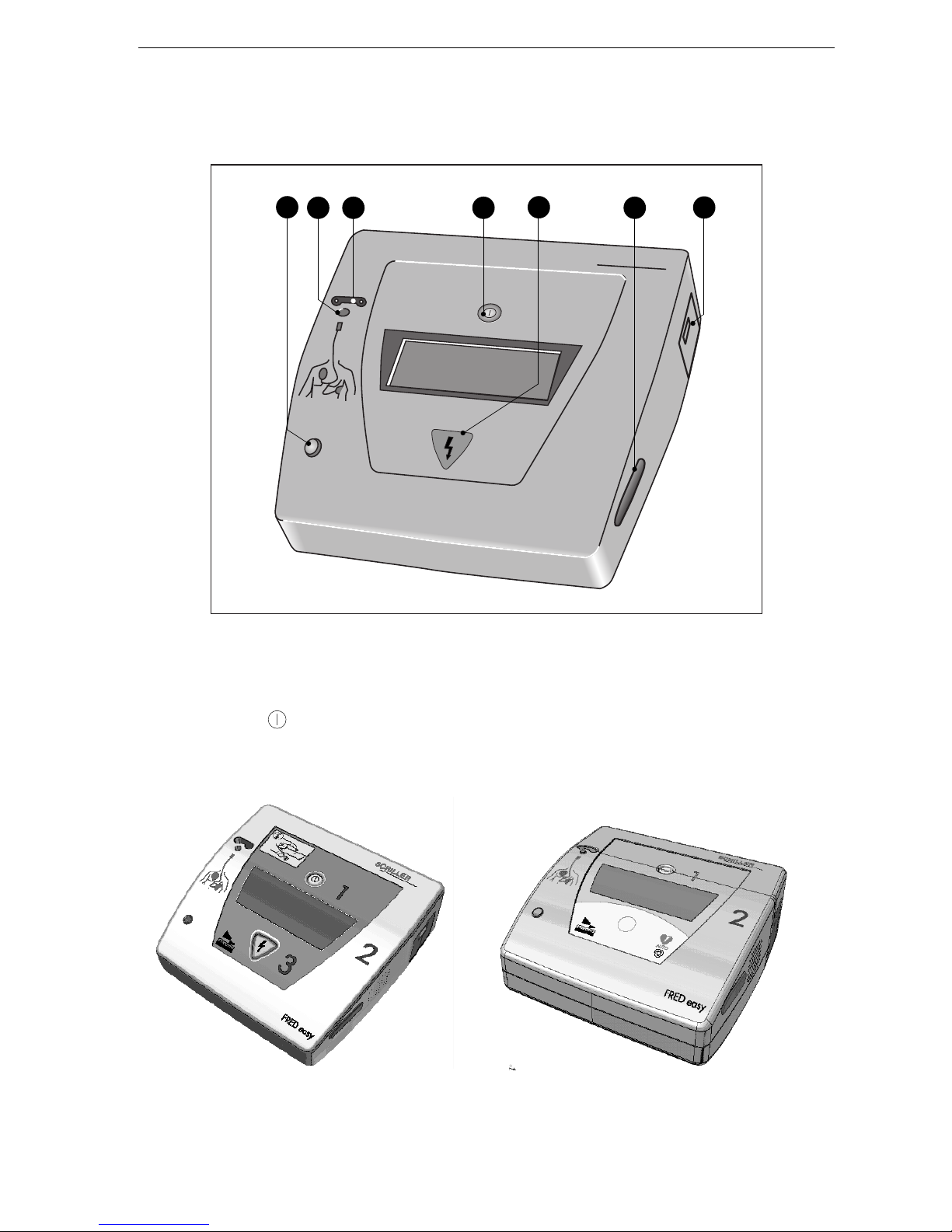
General Information
FRED easy®
4
General Information
•FRED easy®bears the CE mark
CE-0459
indicating its compliance with the provisions
of the Council Directive 93/42/EEC about
medical devices and fulfills the essential re-
quirements of Annex I of this directive. FRED
easy® is a class IIb device.
•The product complies with the electromag-
netic immunity requirements of standard IEC
60601-1-2/EN 60601-1-2 "Electromagnetic
Compatibility - Medical Electrical Equipment".
•The radio interference emitted by this device
is within the limits specified in the CISPR 11
standard.
•The user guide is an integral part of the
device and should always be kept near the
device. Close observance of the information
given in the user guide is a prerequisite for
using the device as intended for and correct
operation and ensures patient and operator
safety. Therefore, be sure to read the
complete user guide.
•To ensure patient safety, the specified meas-
uring accuracy, and interference-free opera-
tion, we recommend to use only original
SCHILLER accessories. The user is respon-
sible if accessories from other manufacturers
are used. The warranty does not cover dam-
age resulting from the use of unsuitable ac-
cessories and consumables from other
manufacturers.
•SCHILLER is responsible for the effects on
safety, reliability, and performance of the de-
vice, only if
−assembly operations, extensions, read-
justments, modifications, or repairs are
carried out by SCHILLER or by persons
authorized by SCHILLER
−the device is used in accordance with the
instructions given in this manual.
•The customer is responsible, if the device is
employed in a manner different from the
method described in this manual.
•On request SCHILLER will provide a detailed
field service manual.
•The manufacturer is only liable for
SCHILLER-supplied accessories.
•The user guide informs the device operator
about the intended use, exact function, op-
eration and required preventive maintenance.
It is not a substitute for a product training.
•The safety information given in this manual is
classified as follows:
Danger
indicates an imminent hazard. If not
avoided, the hazard will result in death or
serious injury.
Warning
indicates a hazard. If not avoided, the
hazard can result in death or serious in-
jury.
Caution
indicates a potential hazard. If not
avoided, this hazard may result in minor
personal injury or product/property dam-
age.
•This manual conforms with the device speci-
fications and safety standards valid at the
time of printing. All rights are reserved for de-
vices, circuits, techniques, software pro-
grams, and names appearing in this manual.
•The SCHILLER quality management system
complies with the international standards ISO
9001 and ISO 13458.
•No part of this manual may be reproduced
without written permission from SCHILLER.
Manufacturer
SCHILLER MEDICAL SAS
4, Rue Louis Pasteur
F-67162 Wissembourg – Cedex, France
Telephone **33 (0) 3 88 63 36 00
FAX **33 (0) 3 88 94 12 82
E-mail info@schiller.fr