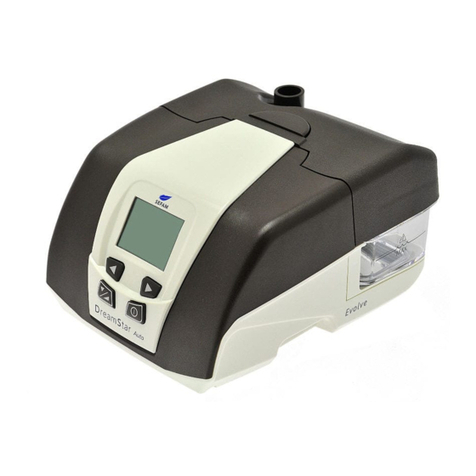
SEFAM EcoStar User manual


2 Table of Contents EcoStar
TABLE OF CONTENTS
Before starting .................................................................................................................................. 3
Safety instructions.......................................................................................................................3
Intended use .................................................................................................................................4
Adverse effects ............................................................................................................................4
Contraindications.........................................................................................................................4
Checking the components .........................................................................................................4
Description of the device.................................................................................................................. 5
Views of the Device .....................................................................................................................5
Symbols on the device................................................................................................................6
Installation......................................................................................................................................... 7
Standard installation of the device ...........................................................................................7
Installing the GoodKnight H2O humidifier ...............................................................................7
Installation for use with battery or cigar lighter power.........................................................7
Use ..................................................................................................................................................... 8
Starting treatment .......................................................................................................................8
Stopping treatment......................................................................................................................8
Mask disconnected feature .......................................................................................................8
Transporting the device..............................................................................................................8
Ramp feature................................................................................................................................9
Accessing information on the device.......................................................................................9
Use with added oxygen (optional)................................................................................................. 12
Installation with an oxygen adapter (optional).................................................................... 12
Starting and stopping treatment............................................................................................ 12
Cleaning and Maintenance ............................................................................................................ 13
Weekly......................................................................................................................................... 13
Monthly....................................................................................................................................... 13
Trouble-shooting............................................................................................................................. 14
Helpful hints............................................................................................................................... 14
Device messages...................................................................................................................... 15
Technical characteristics............................................................................................................... 16
Device performance ................................................................................................................. 16
Conditions of use...................................................................................................................... 16
Electrical characteristics ......................................................................................................... 16
Transport and storage conditions ......................................................................................... 16
Physical characteristics........................................................................................................... 16
Electrical characteristics of the power supply module...................................................... 17
Standards Compliance ............................................................................................................ 17
Disposing of the device at the end of its life........................................................................ 17
CE marking................................................................................................................................. 17

EcoStar Before starting 3
Before starting
Please read this manual carefully before using your device so that you fully understand the limitations
of this device.
Safety instructions
WARNING
In this manual, this signals
a risk of injury or accident
to you or to others.
Only use the EcoStar device for its intended
purpose indicated in this manual. The advice
given in this manual does not replace the
instructions of your health care provider.
The device is not intended to provide
assistance for vital functions.
It must only be used with the circuits, masks,
fittings and accessories recommended by a
physician or supplied by your home care
provider. Confirm the ‘Directions for Use’
instructions for each accessory are in the
package and read them carefully.
When a facial mask is necessary, always use
one which is equipped with an anti-asphyxia
valve.
If you suspect that the device or one of the
accessories is defective, damaged or not
working properly, please contact your home
care provider.
Use only with the power supply module
provided with the device.
Do not try to open or modify the device.
Maintenance of this equipment is to be
performed by skilled personnel only. Contact
your home care provider.
If the device is connected to a base of
multiple sockets, an additional base of
multiple sockets or an extension wire should
not be connected to the system.
If necessary, the device can be detached from
the electrical network by unplugging the
power cord. Make sure that the power cord is
accessible.
Place the device on a stable horizontal
surface in a clean, dry environment. Keep the
device away from sources of water.
Keep the device away from children and pets
or pests.
In the event of additional oxygen supply,
scrupulously observe the safety instructions
for using oxygen.
Once the mask is in place, make sure that the
device produces airflow. If not, remove the
mask immediately and contact your home
care provider.
Be careful not to obstruct the air outlet,
or any other opening of the device or
respiratory circuit, either accidentally or
intentionally. Avoid covering the device or
placing it too close to a wall. Do not introduce
liquids or objects into the air outlet.
Never block the mask exhalation vent, which
allows air to be expelled continually and
reduces to a minimum the re-inhalation of
carbon dioxide. When the CPAP device is
turned on and is functioning properly, fresh air
from the CPAP device flushes the exhaled air
out through the interface vent hole. However,
when the device is not operating, insufficient
fresh air is produced in the mask presenting a
risk of re-inhaling exhaled air, which under
certain circumstances could lead to a risk of
suffocation within several minutes.
At lower CPAP pressures, the flow through the
exhalation port may be inadequate to clear all
exhaled gas from the hose. There may also be
a risk of re-inhalation.
If the device experiences a loss of power or a
malfunction, remove the mask.
Do not leave long pieces of unused tubing on
the bed, to prevent them from wrapping
around your head or neck while you sleep.
CAUTION
In this manual, this indicates
that there is a possibility of
material damage to this
device or any other device.
The EcoStar device cannot be used without a
medical prescription. Under no circumstances
should you attempt to adjust these prescribed
settings without the agreement of your
medical team.
Since this is a medical electrical device, please
follow the installation instructions contained in
this manual concerning electromagnetic
compatibility indicated by your home care
provider.
Like all medical electrical devices, the device is
vulnerable to interference from mobile and
portable radiofrequency communication
equipment (cordless phones, WiFi) which could
be placed nearby, except in the case of use
with the GoodKnight H2O humidifier.

4 Before starting EcoStar
If the device is used with the GoodKnight H2O
heating humidifier:
Refer to the safety instructions in the user
manual for your humidifier.
When using the humidifier's water chamber,
take precautions to eliminate the risk of
introducing water into the device, which can
cause irreversible damage. Make sure that the
humidifier is always lower than the device.
Fill the water chamber away from the device
to avoid spilling water on the device before
connecting it to the humidifier.
Place the heating humidifier and the EcoStar
device on a flat, stable surface and keep them
away from sources of flames.
Never put the heating humidifier on top of the
device as water could enter the device and
damage it.
After use, disconnect the humidifier from the
device to avoid exposing the device to
humidity.
Adding a humidifier may affect the device
performance.
Disconnect the device from the heating
humidifier and empty the water chamber
before moving or transporting the assembly.
Intended use
The EcoStar device is a Positive Pressure
device indicated for the mask treatment of
Sleep Apnea Syndrome (SAS) in patients
weighing more than 30 kg (66 lbs). It can be
used at home or in a sleep center.
The device can be used with the GoodKnight
H2O humidifier if a prescription for heated
humidifi-cation has been added to the patient's
treatment. The heated humidifier is designed
to heat and raise the humidity of the air
delivered to the patient through the Continuous
Positive Airway Pressure (CPAP) device.
Adverse effects
Please contact your health care professional if
you develop the follow symptoms when using
the device: unusual thoracic pains, severe
headache, dyspnea increase, airway or nasal
passage dryness, skin sensitivity, runny or
bleeding nose, discomfort or pain in ear or
sinus, bloating, daytime drowsiness, mood
changes, disorientation, irritability or memory
loss.
Contraindications
Studies have shown that the use of positive
airway pressure is contraindicated for
certain patients with one of the following
pre-existing conditions:
Severe bullous emphysema or
emphysema previously complicated by
pneumothorax.
Pneumoencephalus, trauma or recent
surgery with sequela of cranio-
nasopharyngeal fistula.
Decompensated cardiac insufficiency or
hypotension, particularly in case of
decreased blood volume or cardiac
arrhythmia.
Dehydration.
Massive epistaxis or history of massive
epistaxis.
Acute sinusitis, otitis media, or
perforated tympanic membrane.
Tracheotomy.
Checking the components
The device is delivered with the following
components:
Power supply
Air inlet filter
Carrying case
Patient circuit
Patient manual
It can also be used with the following optional
accessories. For more information about
available accessories, contact your home
care provider. When using the accessories,
follow the instructions provided.
GoodKnight H2O

EcoStar Description of the device 5
Description of the device
The EcoStar
device is powered by an external electrical power supply module and comes with
specific accessories
Views of the Device
Figure 1 View of the user interface
1
Display
this allows viewing information such as the pressure delivered
and the device settings.
2
Information access
button
this allows access to information about the device.
3
Ramp button
this allows disabling the pressure ramp. It is also used to
decrease the value of the parameters during the device settings.
4
On/Off button
this allows turning on or off the device. It is also used to increase
the value of the parameters during the device settings.
Figure 2 View of the front side
5
Outlet connector
air outlet to which the tubing is connected.
5
1
3
2
4

6 Description of the device EcoStar
Figure 3 View of the rear side
6
Supply connector
this allows powering the device by the power supply module or by
an external battery.
7
Air inlet filter
prevents dust from entering the device and the airflow.
Symbols on the device
Symbol
Description
Symbol
Description
On/off button symbol.
Ramp button symbol.
Symbol for raising the value of the
parameter displayed on the screen.
Symbol for lowering the value of the
parameter displayed on the screen.
Information access button symbol.
Outlet symbol.
Device is protected against the
penetration of solid objects larger
than 12 mm and against falling
drops of water.
Device at the end of its life, separate
from household waste for disposal.
For further information, refer to
"Disposing of the device at the end
of its life" on page 17.
Class II device.
BF-type device.
Direct current power supply.
Direct current.
Refer to the user manual.
Specific warning (see "Safety
instructions" on page 3).
Device complies with the
requirements of European Directive
93/42/EC on medical devices.
Keep dry.
6
7

EcoStar Installation 7
Installation
Standard installation of the device
1. Connect the end of the tubing to the
outlet connector of the device (Item 5 of
Figure 2 on page 5).
2. Prepare the mask as described in the
operating instructions which came with
the mask.
3. Connect the mask to the other end of
the patient circuit.
4. Connect the power supply module cord
into the supply connector on the rear
side of the device (Item 6 of Figure 3 on
page 6) and the power supply module
plug into the power outlet.
5. "SEFAM" is displayed, followed by the
next standby screen. The device is ready
to operate.
Installing the GoodKnight H2O
humidifier
Please see your GoodKnight H2O's user
manual to prepare the humidifier and
proceed with installation on your device.
Then continue with steps 2 through 5 of the
standard installation procedure.
Installation for use with battery or
cigar lighter power
The EcoStar device can be powered by a 12-
Volt battery by connecting one end of the
specific optional cable to the supply
connector on the rear side of the device
(item 6 of Figure 3 on page 6) and the other
end directly to the battery.
The device also can also be powered from a
cigarette-lighter socket using an optional
cable specially designed for this purpose. To
do this, connect one end of the cigarette-
lighter cable to the supply connector on the
rear side of the device (Item 6 of Figure 3 on
page 6) and the other end directly to the
cigarette-lighter socket.
CAUTION
Do not use a battery power cable other than
the one intended with the device. There
would be a risk of damaging the device and
your battery.
Use only a 12-volt DC power source and
observe the correct connection polarity
(+and -).

8 Use EcoStar
Use
Starting treatment
1. Put the mask in place according to the
instructions which came with the mask. If a
mask with exhalation vent is used, it
includes a hole by which the exhaled gases
will be flushed and could not be rebreathed.
If the mask does not have an exhalation
vent, your physician must provide you with
an attachment for expelling air as closely
as possible to the nose.
2. Press the on/off button to begin
treatment. The prescribed pressure or the
actual pressure is displayed on the screen,
according to the setting that you carried
out.
3. If you use a heating humidifier, turn it on
according to the instructions which came
with the humidifier.
4. The symbols which may be displayed on
the screen are summarized in the table
entitled "Description of symbols displayed
on the screen" on page 10.
WARNING
Following a power supply interruption, the device
will resume the same mode it was in (on/off) before
the disconnection.
Stopping treatment
1. If you use a heating humidifier, turn it
off according to the instructions which
came with the humidifier. Always
unplug the humidifier before turning off
the device.
2. Remove the mask.
3. Press the on/off button to turn
the device off. The device will go into
standby mode and display "Eco". You
can then unplug the power supply
module cord from the power outlet.
Mask disconnected feature
If you remove your mask, the device
automatically switches to low power. The
machine will restore normal power when
you reconnect you mask, or it will stop if
you press the on/off button .
This feature can be used at night if you
need to get out of bed.
Transporting the device
Unplug the power supply module and
disconnect all the device's accessories.
Place the power supply module, the
accessories and the device in the carrying
case.
CAUTION
Disconnect the device from the GoodKnight
H2O heating humidifier and empty the water
chamber before moving or transporting the
assembly, to eliminate any risk of introducing
water into the device and thereby causing
irreversible damage.

EcoStar Use 9
Ramp feature
If activated by your home care provider, this
feature allows for a gradual rise in pressure
to help you go to sleep: therefore, treatment
begins at a low pressure called the comfort
pressure, and rises from there to the
pressure for treatment.
The pressure rise time is prescribed by your
medical team and is set by your home care
provider.
1. Press the on/off button to start the
ramp. The ramp indicator is
displayed.
Note:
If the ramp time is set at zero, the ramp
feature is disabled.
2. Press the ramp button to turn the
ramp feature off. You can reactivate it
by stopping and restarting the device by
pressing the on/off button twice.
The value of the comfort pressure can be
changed. To do that, refer to the "Device
setting" paragraph on page 10.
Accessing information on the
device
The three buttons on the front of the device
allow access information on the device and
are used to change the value of the
parameters for certain settings.
The parameters accessible on the screen
when the machine is in standby or operating
mode are:
parameters of the device relating to your
treatment
the compliance data which has been
recorded.
Each screen of the patient settings menu
includes:
an upper section indicating the value of
the parameter displayed
a lower section which includes various
symbols indicating whether the machine
is on or off, as well as the nature of the
parameter displayed (see table entitled
"Description of symbols displayed on the
screen" on page 10).

10 Use EcoStar
Description of symbols displayed on the screen
Symbol
Description
Symbol
Description
Standby mode
Operating mode
Ramp feature
Pressure symbol
Time symbol
Compliance symbol
Adjustable parameter
Software version
Information message
Error message
Device setting
The display allows information for the device's settings to be viewed. The information is available
whether the device is on standby or in use.
To access the device settings:
press the information access button for one
second.
On the screen which appears:
the symbol or the symbol shows if the
device is on standby or in use.
the symbol indicates that the parameter
displayed can be modified. Raise the value of the
parameter by pressing the on/off button or
lower it using the ramp button .
To access the next data:
press the information access button .
To exit the parameter settings menu:
press the information access button again.
The display will indicate "Eco" (in standby mode) or
the prescribed pressure (in operating mode).
Notes:
Exiting the parameter setting menus happens automatically if you do not press any buttons for 30
seconds.
In the information access sequence below, the device is shown in operating mode and all the values
displayed are given by way of example.

EcoStar Use 11
❶
This screen appears:
Press
for one
second.
Pressure set by the physician (corresponding
to the pressure prescribed when the device is
operating).
❷
Press
Ramp time set by the physician (time the
device takes to reach the prescribed pressure
when starting from the comfort pressure).
❸
Press
Comfort pressure (level of pressure produced
by the device when the ramp feature starts up);
this parameter is only displayed if the ramp
time is not 0. The symbol indicates that you
can raise or lower the value displayed by using
the button or .
❹
Press
Value of the hour counter (device operating
time). The value displayed on the right below
represents the minutes.
❺
Press
Value of the compliance counter (time during
which you actually breathed with the mask).
The value displayed on the right below
represents the minutes.
❻
Press
Selection of the pressure displayed: prescribed
pressure Pd or actual pressure Pr (estimated at
the mask). The selection is done by pressing
the button .
❼
Press
Software version included with the device.
The version number is indicated by two digits
(); for example, the screen might display:
Id 1.0.

12 Use with added oxygen (optional) EcoStar
Use with added oxygen (optional)
WARNINGS
When using oxygen, always follow the
instructions of the medical team or home
care provider. The source of oxygen
should be placed at over one meter of the
device.
Do not smoke in the presence of oxygen.
Do not inject oxygen into the device’s air
intake.
Carefully follow the instructions of the
"Starting and stopping treatment"
paragraph.
If you use an oxygen concentrator or
liquid oxygen unit, stop the flow of oxygen
when the device is not operating. If the
oxygen concentrator remains on when
the device is turned off, the oxygen
delivered into the patient circuit could
accumulate inside the device, creating a
fire hazard.
The maximum flow of oxygen used must
not exceed 12 l/min.
CAUTION
For a given oxygen flow, the concentration of
oxygen inhaled varies as a feature of the
pressure settings, your breathing, the type of
mask used and the leak rate. This precaution
applies to most Continuous Positive Airway
Pressure devices.
Installation with an oxygen adapter
(optional)
When using an additional oxygen supply, you
must use a valve designed to prevent the
accumulation of oxygen in the device.
This valve must be installed between the
device and the patient circuit.
Refer to manufacturer's instructions for
installation, cleaning and maintenance of the
valve.
Starting and stopping treatment
1. It is essential that the device is turned on
and generating air flow before the oxygen
flow is started so that oxygen does not
accumulate in the EcoStar device.
2. Likewise, you must stop the oxygen flow
before turning off the device so that
oxygen does not accumulate in the
EcoStar device.

EcoStar Cleaning and Maintenance 13
Cleaning and Maintenance
Please refer to the instructions for your mask, the heating humidifier and the respiratory circuit for
more information about the care and maintenance of these items.
WARNING
Always unplug the device from the electrical source and disconnect the respiratory circuit from the
device before cleaning.
CAUTION
Use appropriate materials for cleaning: do not use harsh detergents, abrasive sponges or brushes with
hard bristles.
Do not let water come into contact with the device.
Weekly
Air inlet filter
Remove the filter at the rear of the device.
Wash the filter with warm water and mild
detergent (for example 1 drop of
dishwashing liquid on the filter).
Rinse well to eliminate any trace of
detergent.
Dry the filter:
oPress dry in a clean, absorbent
cloth
oAllow to dry completely away
from sunlight
Once dry, reinstall the filter at the rear of
the device. Do not use a filter which is not
completely dry.
Monthly
Device
Clean the device exterior regularly by
using a damp cloth or paper towel
moistened with a little water and a
drop of gentle detergent.
Remove detergent residue by
repeating this step with a clean cloth
or paper towel, slightly moistened with
just water.
Wipe the entire device with a dry cloth
or paper towel.
Air inlet filter
Change the filter whenever it is damaged
or soiled.
WARNINGS
Do not use detergent sprays. Chemical
product residue could enter the air outlet,
the filter's foam or the device interior,
causing airway irritation.
Never use the device without making sure
that an air inlet filter is installed.

14 Trouble-shooting EcoStar
Trouble-shooting
Helpful hints
Problem
Possible cause
Solution proposed
Your nose is cold.
The room temperature is
too low.
The delivered air is too
cold.
Raise the room temperature.
Place patient circuit under a blanket to
reduce heat loss.
Runny Nose.
Reaction to the airflow and
pressure.
Contact your medical technical team or your
attending physician.
Nose or throat is dry
or irritated.
The air is too dry.
Humidify the air in the room using a
humidifier.
Contact the medical technical team to
obtain a heating humidifier.
Nose, sinus or ear
pain.
Sinus infection or nasal
congestion.
Contact the attending physician
immediately.
The device is
delivering too hot air.
The air inlet filter may be
dirty. The air inlet is
clogged.
Room temperature is too
high.
Clean or replace filters as necessary (see
chapter entitled “Cleaning and Maintenance”
on page 13). Move the device away from all
linens and clothing.
Lower the room temperature. Make sure
that the device is well away from any source
of heat. Remove patient circuit from
underneath the blanket.
Discomfort from
feeling that the
pressure is too high.
Device pressure.
Getting used to the nasal pressure takes
some time. Use the pressure ramp when
you go to sleep (see "Ramp feature”, page 8).
The pressure level will gradually increase
before reaching the prescribed value and the
ramp indicator will be displayed. Relax and
breathe slowly through the nose.
The pressure level has been prescribed by
your physician; it can be changed only by
medical prescription. If the pressure
delivered by the device seems to have
changed, contact your home care provider
to have the device pressure checked.
The device does not
display the correct
pressure.
The ramp is enabled.
Confirm that the ramp indicator is displayed.
Disable the ramp feature by pressing the
ramp button.

EcoStar Trouble-shooting 15
Problem
Possible cause
Solution proposed
The device does not
light up (no display).
The power supply module
is not properly connected.
No mains power.
The device's internal fuse
is defective.
Check the connections between the device,
the power supply, and the power source.
Plug another device (e.g., lamp, radio, etc.)
into the power source to confirm that current
is available at the source.
Contact your home care provider.
The device seems to
be experiencing
interference and is
not operating
properly.
Excessive
electromagnetic
interference.
Move the device away from sources of
interference, such as halogen lamps, cordless
phones, etc.
CAUTION
If other problems occur, contact your home care provider.
Device messages
Message displayed
Description
Solution proposed
In 01
The mask is
disconnected.
Check the connections between the mask,
the patient circuit, and the device. This
message disappears as soon as the mask is
well connected.
In 02
The device has detected
excess pressure for more
than 10 seconds.
Contact your home care provider.
In 03
Reduction of the power
supply voltage.
Check the connections between the power
supply module, the device and the power
outlet. Unplug the power supply module, then
reconnect it to the power outlet. If the
problem persists, contact your home care
provider.
Check the battery and replace it if necessary.
If the message persists, contact your home
care provider.
Er XX
(XX = 2 digits).
The device has detected
an operating fault.
Contact your home care provider.

16 Technical characteristics EcoStar
Technical characteristics
Device performance
Device pressure range:
4 cmH2O to 20 cmH2O 1 cmH2O
device adjustable in increments of 0.5 cmH2O
Maximum pressure at the patient-side
connection port under single fault condition:
30 cmH2O
Ramp time:
0 to 30 minutes 1 minute
device adjustable in increments of 5 minutes
Patient-side connection aperture:
Conical connection of 22mm diameter.
Sound pressure level measured in accordance
with NF EN ISO 17510-1: 2002:
27 dB(A)
Sound pressure level measured in accordance
with NF EN ISO 17510-1: 2009:
29 dB(A)
Lifetime planned for the device:
5 years (8 hours per day)
Conditions of use
Electrical characteristics
Absolute pressure
range:
700 hPa to 1060 hPa
Maximum power
consumption:
20 W
Temperature:
+5°C to +40°C
(+41 °F to +104 °F)
Input voltage:
13 V
+5°C to +35°C
(41 °F to 95°F) with
GKH2O humidifier
Current consumed
at 20 cmH2O with a
4 mm leak:
0.750 A.
Relative humidity:
between 10% and 95%
without condensation
Altitude range:
0 2,400 m approx.
(7,900 ft approx.)
Transport and storage conditions
Physical characteristics
Relative pressure
range:
500 hPa to 1060 hPa
Dimensions
(D x W x H):
202 x 145 x 79 mm approx.
(7.9 x 5.7 x 3.1 ins approx.),
excluding the power supply
module
Temperature:
-20°C to +60°C
(-4 °F to +140 °F)
Weight:
0.644 kg approximately
(1.4 lbs approx.), excluding
the power supply module
Relative humidity:
up to 95% without
condensation

EcoStar Technical characteristics 17
Electrical characteristics of the power supply module
Class II power supply:
Input voltage: 100 –240 VAC (-15%, +10%), 50/60 Hz (1 Hz)
Power supply module provided
Input current
Output voltage
NEWTIM SNT-M1601 (EU plug)
1 000 mA
13 V / 1.80 A
DELTA MEF-023A13C (EU plug)
600 - 800 mA
13 V / 1.80 A
POWERWIN PW-M024A-1Y120K
(plug except EU)
600 mA
12 V / 2 A
WARNINGS
Use only one of the above-mentioned specific power supplies provided with the device.
The above-mentioned power supply modules are not intended to be repaired. If it stops working, please
contact your home care provider for a replacement.
Standards Compliance
Disposing of the device at the
end of its life
Risks pertaining to this medical equipment were
assessed in accordance with the ISO 14971: 2007
standard, specifically with reference to global residual
risk. The EcoStar device complies with the following
directives and standards:
IEC 60601-1:2005 + Amd1:2012: Medical electrical
equipment. Part 1: General requirements for basic
safety and essential performance.
IEC 60601-1-2:2014: Medical electrical equipment
–Part 1-2: General requirements for basic safety
and essential performance –Collateral Standard:
Electromagnetic disturbances –Requirements
and tests.
ISO 80601-2-70-1:2015: Medical electrical
equipment -- Part 2-70: Particular requirements for
basic safety and essential performance of sleep
apnea breathing therapy equipment.
NF EN ISO 5356-1:2005 : Anaesthetic and
respiratory equipment. Conical connectors.
Council Directive 93/42/EC concerning medical
equipment.
European Parliament and Council Directive
2011/65/EC on the restriction of the use of certain
hazardous substances (RoHS) in electrical and
electronic equipment.
European Parliament and Council Directive
2012/19/EC on waste electrical and electronic
equipment (WEEE).
In compliance with European Directive
2012/19/EC this device is an electrical
and electronic piece of equipment and
must be collected and processed
separately from household waste for
disposal.
The symbol of the crossed out garbage
bin (see paragraph "Symbols on the
device", on page 6) indicates that this
equipment must be collected and
handled using an appropriate method of
disposal.
Unsuitable disposal of the device at the
end of its life could harm the
environment.
Contact your home care provider.
CE marking
EcoStar : 2013.

18 Technical characteristics EcoStar


Manufacturer:
Manufacturing plant:
SEFAM
144 AV CHARLES DE GAULLE
92200 NEUILLY SUR SEINE
FRANCE
SEFAM
10 ALLEE PELLETIER DOISY
54600 VILLERS-LES-NANCY
FRANCE
: M-159DFU00-20 Version 6
2019-01
Home care Provider Information
Other manuals for EcoStar
1
Table of contents
Other SEFAM Medical Equipment manuals
Popular Medical Equipment manuals by other brands

NSK
NSK Primado2 Operation manual

Neurosoft
Neurosoft Neuro-Audio-Screen/OAE Technical manual

Medical International Research
Medical International Research Spirolab user manual
imtmedical
imtmedical bellavista Quick start manual

Edwards Lifesciences
Edwards Lifesciences 3000TFX PERIMOUNT Magna Aortic Instructions for use

GCE druva
GCE druva MEDIUNIT Instructions for use