Neurosoft Neuro-Audio-Screen/OAE User manual
Technical Manual
Neuro-Audio-Screen
Neuro-Audio-Screen/OAE
Portable All-in-One ABR, DPOAE&TEOAE
Hearing Screening Systems
TM057.02.002.00
0
(
14
.
1
1
.20
1
2
)

Neurosoft Ltd. © 2013
5, Voronin str., Ivanovo, 153032, Russia
P.O. Box 10, Ivanovo, 153000, Russia
Phone: +7 (4932) 24-04-34 Fax: +7 (4932) 24-04-35
E-mail: com@neurosoft.ru Internet: www.neurosoft.ru

3
Table of Content
Introduction............................................................................................................... 5
List of Abbreviations ................................................................................................ 6
1. Important Safety Instructions ........................................................................... 7
2. Description and Operation................................................................................ 9
2.1. Neuro-Audio-Screen Function....................................................................... 9
2.1.1. Otoacoustic Emission and its Recording............................................... 9
2.1.2. Auditory Brainstem Response and Its Recording................................ 10
2.2. Main Specifications..................................................................................... 11
2.3. Arrangement and Operation........................................................................ 14
3. Description and Delivery Set .......................................................................... 16
4. Mounting and Setting ...................................................................................... 19
4.1. Unpacking and Check of Delivery Set......................................................... 19
4.2. Room Selection and Placement.................................................................. 19
4.3. Requirements to the Personnel Conducting Mounting and Setting.............. 19
4.4. Getting Started............................................................................................ 20
5. Functioning ...................................................................................................... 21
5.1. Control and Indication Tools ....................................................................... 21
5.1.1. Control Buttons................................................................................... 21
5.1.2. Indication............................................................................................ 22
5.1.2.1. Display Means ........................................................................... 22
5.1.2.2. Displaying of Main Parameters .................................................. 22
5.1.3. Connectors......................................................................................... 24
5.2. Turning on .................................................................................................. 25
5.3. Rechargeable Battery Charge..................................................................... 25
5.4. Entering of Personal Data of New Patient, Selection of Patient from
Database, Removal of Patient Data............................................................ 29
5.5. Patient Preparation for OAE Test................................................................ 31
5.6. TEOAE Test ............................................................................................... 34
5.7. DPOAE Test ............................................................................................... 37
5.8. Patient Preparation for ABR Test................................................................ 40
5.9. ABR Test .................................................................................................... 41
5.10. Review, Printing and Removal of Results ................................................. 44
5.10.1. Review and Printing of Test Results ................................................. 45
5.10.2. Removal of Test Results................................................................... 48
5.10.3. TEOAE Report Description............................................................... 49
5.10.4. DPOAE Report Description .............................................................. 50
5.10.5. ABR Report Description.................................................................... 52
6. Settings ............................................................................................................ 53
6.1. General Settings......................................................................................... 55
6.2. TEOAE Settings.......................................................................................... 56
6.3. DPOAE Settings ......................................................................................... 57
6.4. ABR Settings .............................................................................................. 59
6.5. System Settings.......................................................................................... 60
7. Data Exchange with Computer ....................................................................... 65
7.1. Screening System Connection to Computer ............................................... 65
7.2. Operation with Neuro-Audio-Screen Manager Software.............................. 69
7.2.1. Receiving of Patient List and Exams .................................................. 72
7.2.2. Operation with Saved Exams ............................................................. 74

4
7.2.3. Exam Review and Print ...................................................................... 75
7.2.4. Operation with Patients List................................................................ 75
7.2.5. Service Functions............................................................................... 78
7.2.6. Firmware Updating ............................................................................. 79
8. Troubleshooting .............................................................................................. 82
9. Technical Servicing ......................................................................................... 84
9.1. General Requirements................................................................................ 84
9.2. Maintenance Works .................................................................................... 84
9.3. Cleaning and Desinfection .......................................................................... 84
9.4. Device Functioning Check Using Test Cavity.............................................. 85
9.5. OAE Probe Check ...................................................................................... 86
9.6. Conservation .............................................................................................. 90
10. Packing and Transportation ........................................................................... 90
11. Storage Regulations........................................................................................ 91
12. Utilization of Neuro-Audio-Screen and Neuro-Audio-Screen/OAE............... 91
13. Delivery Set and Package Data....................................................................... 91
14. Acceptance Certificate .................................................................................... 92
15. Delivery Certificate .......................................................................................... 92
16. Storage Data .................................................................................................... 92
17. Warranty........................................................................................................... 93
18. Reclamation Data ............................................................................................ 94
19. Repair Data ...................................................................................................... 96
Annex 1. Electromagnetic Emission and Immunity.............................................. 97
Annex 2. Format of Neuro-Audio-Screen Exported Files................................... 101
Introduction
5
Introduction
This technical manual (hereinafter referred to as “manual”) is the combined document
describing the operation and the servicing of Neuro-Audio-Screen portable all-in-one
ABR, DPOAE&TEOAE hearing screening system (hereinafter referred to as screening
systems) intended for the hearing examination of human being including the
newborns using otoacoustic emission and auditory brainstem response techniques.
The manual is the document certifying the technical parameters of the products which
are guaranteed by the manufacturer.
Read carefully this technical manual before starting to work!
You can send your responses and recommendations to the following address:
P.O. Box 10, Ivanovo, 153000, Russia
or by e-mail:
help@neurosoft.ru.
You can find additional information on Neurosoft products in the Internet:
www.neurosoft.ru
or ask questions by phones:
+7 (4932) 24-04-37 (Service department),
+7 (4932) 24-04-34.

Hearing Screening Systems (Technical Manual)
6
List of Abbreviations
ABR – auditory brainstem response
BERA – brainstem evoked response audiometry
DPOAE – distortion product evoked otoacoustic emission
IHC – inner hair cells
LCD – liquid-crystal display
OAE – otoacoustic emission
OHC – outer hair cells
SPL – sound pressure level
TEOAE – transient evoked otoacoustic emission

Important Safety Instructions
7
1. Important Safety Instructions
Neuro-Audio-Screen and Neuro-Audio-Screen/OAE is designed to be used only by
those individuals trained to perform the testing for which it has been designed. No
person should attempt to use these hearing systems without the necessary
knowledge and training to understand how this equipment is to be properly utilized
and interpreted.
The hearing system probe must not be inserted into an ear without an ear
tip properly affixed.
The hearing systems do not contain high-voltage circuits inside which can represent a
danger for a human being. The hearing systems ensure the safe operation at the
correct exploitation. However, the number of precautions at the hearing systems use
exists:
Do not discharge the battery completely. When storing the discharged Li-ion battery
degrades fast over time.
Do not immerse the device in water or any other solutions (for example, do not
leave it near the aquarium in the presence of a child). See the device cleaning pro-
cedures in section 9 “Technical Servicing” of this manual.
Use and store the instrument indoors only. Do not expose this device or its
accessories to temperatures below 5ºC or above 40ºC, or to relative humidity of
more than 90%.
Do not drop or otherwise cause undue impact to this device. If the screening
system is dropped or otherwise damaged, return it to the manufacturer for repair
and/or calibration. Do not use the screening system if any damage is suspected.
Do not attempt to open or service the screening system. Return the device to the
manufacturer or send to the company authorized by the manufacturer for all
service. Opening the screening system case will void the warranty.
Do not operate the printer if the power supply has a damaged cord or plug.
Do not expose the printed results to sunlight or heat. Printing on thermal paper
fades with exposure to light or heat.
Photocopies of test results should be made if the records are to be kept indefinitely.
The equipment and accessories used together with the device should satisfy the
requirements stated in the present manual (table 2 and table 3 “Document code or
main specifications” column). The violation of this requirement can impact nega-
tively the electromagnetic compatibility (result in the increase of emissions or
reduction of immunity) and also the safety and functioning of the screening system.

Hearing Screening Systems (Technical Manual)
8
Precautions at Operation with Printer and Screening System AC Power Supply
The power supply unit of Neuro-Audio-Screen and Neuro-Audio-Screen/OAE
screening systems transforms the mains voltage (220/230 V 50/60 Hz) into the direct
voltage (9 V) and is intended for the charge of the rechargeable battery built in the
device and the screening system power supply only in case the battery is discharged.
The printer power supply unit is used ONLY for the printer power supply and has the
specifications differing from the Neuro-Audio-Screen and
Neuro-Audio-Screen/OAE power supply unit. The connector of printer power supply
unit of different manufacturers can coincide with the device power supply connector. It
can cause a mistake at the power supply unit connection that is why, please, be
attentive. The use of the printer power supply unit for the supply of Neuro-Audio-
Screen and Neuro-Audio-Screen/OAE screening systems can result in the device
failure and the rechargeable battery outage.
Do not connect the printer power supply unit to Neuro-Audio-Screen and
Neuro-Audio-Screen/OAE screening systems.
It is prohibited to use the power supply of other type and manufacturer to supply the
device as it can result in the device failure or a patient’s electrical shock.
The power supply is intended for the operation from the AC power supply with
220/230 V 50/60 Hz voltage, do not insert the unit into the 380/400 V outlet.
The power supply is for indoor use only. Do not expose to water or excessive dust.
The power supply is not intended for the operation in the presence of the highly
inflammable mixture of anesthetics with an air or nitrous oxide.
Do not cover the power supply body as it may result in excessive heating.
The power supply operates when the plug is inserted into an outlet. To turn
it off, remove the plug from the outlet. The outlet must be easily accessible
and located near the printer. Should a faulty condition occur, remove the
plug from the outlet immediately.

Description and Operation
9
2. Description and Operation
2.1. Neuro-Audio-Screen Function
The Neuro-Audio-Screen and Neuro-Audio-Screen/OAE hearing screening
systems are intended for the objective audiometry.
The devices can be applied in patient care institutions, diagnostics centers, maternity
hospitals, neuro-surgical clinics and experimental laboratories of the research
institutions for:
the study of the auditory brainstem response (ABR) (only Neuro-Audio-Screen)
(also known as brainstem evoked response audiometry (BERA));
the study of the otoacoustic emission (OAE) using TEOAE and DPOAE techniques.
The screening systems allow choosing a exam type, entering a patient’s data, controlling
a exam process, results displaying and a recorded exams database. It is possible to
process and archive the exam results on the computer, print them using the printer
connected via the wireless interface.
2.1.1. Otoacoustic Emission and its Recording
What is TEOAE?
Transient evoked otoacoustic emission (TEOAE) is an acoustic signal that can be
detected in the ear canal of a person with normal outer hair cell (OHC) function,
subsequent to stimulation of the auditory system with a series of wideband clicks.
What is DPOAE?
Distortion product otoacoustic emission (DPOAE) is an acoustic signal that can be
detected in the ear canal of a person with normal outer hair cell function, subsequent
to stimulation of the auditory system with a pair of pure tones at frequencies f1 and f2.
The resulting emission of interest is the distortion product tone at the
frequency 2 f1-f2.
What Do Otoacoustic Emission Results Tell Us?
Available evidence suggests that otoacoustic emission (OAE) is generated by the
cochlea’s outer hair cells, and that the presence of OAE is an indication that the outer
hair cells are normal. Although OAE test data provide no indication of inner hair cell
(IHC) function, or of hearing ability, current research indicates that the majority of
hearing-impaired individuals will be identified by a simple OAE test. Patients who fail
to generate OAE should be re-screened and/or referred for additional audiological
testing.

Hearing Screening Systems (Technical Manual)
10
How Does Screening Systems Record TEOAE?
The Neuro-Audio-Screen and Neuro-Audio-Screen/OAE screening systems gener-
ate a series of clicks using a telephone, direct them into the ear canal, and then
average the response received by the microphone built in the probe recording OAE.
Owing to the use of the fast Fourier transform for the spectral analysis and the
bandpass filters, the devices provide an estimate of outer hair cell function over a
wide range of frequencies.
How Does Screening System Record DPOAE?
The Neuro-Audio-Screen and Neuro-Audio-Screen/OAE screening systems
generate a series of test tones using two telephones, direct them into the ear canal,
and then measure the level of the DPOAE tone generated by the cochlea by the
microphone built in the probe. By using different test frequencies, the devices provide
an estimate of outer hair cell function over a wide range of frequencies.
2.1.2. Auditory Brainstem Response and Its Recording
What is ABR?
Auditory brainstem response (ABR) is an electrical signal recorded from the
electrodes placed on the head of a patient with the normally functioning organs of
hearing including the auditory nerve and projection areas of the cerebral cortex. This
signal is the result of the auditory analyzer stimulation by the series of the auditory
pulses.
What Do ABR Test Results Tell Us?
As far as ABR is generated by the cochlea, the auditory nerve and the projection
areas of the cerebral cortex, we can safely say that the ABR presence indicates the
normal functioning of all the above mentioned auditory system parts and a patient
hears an acoustic signal directed to the ear canal.
How Does Neuro-Audio-Screen Screening System Record ABR?
The Neuro-Audio-Screen screening system generates a series of clicks using a
telephone, directs them into the ear canal, and then averages the electrical signal
received from the electrodes placed on a patient’s head. As a result ABR is obtained.
Owing to the high frequency of stimulus delivery (up to 93 Hz), the device can register
the so called steady-state potentials. These potentials differ in the high amplitude, are
easily and quickly separated, practically not influenced by the external
electromagnetic fields.
Description and Operation
11
2.2. Main Specifications
Table 1. The Screening System Specifications
Parameters Values
ABR Technique
Recorded EP range 0.15–900 µV
Common-mode rejection not less than 100 dB
Noise level (rms) not more than 0.35 µV
Amplifiers input impedance not less than 90 MΩ
Amplifiers input capacity not more than 40 pF
Differential input bias voltage, maximum permissible (30030) mV
Bandpass flatness in the range from 200 up to 3000 Hz -305%
Electrode impedance range 0.5–500 kΩ
Stimulus intensity range for OAE probe 0-60 dB HL
Stimulus intensity range for TDH-39 headphones 0–100 dB HL
Stimulation frequency range 9–93 Hz
with 1 Hz step
TEOAE Technique
Frequency range 400–5000 Hz
Stimulus intensity range 50–90 dB SPL
Stimulus spectrum flatness
in the frequency range 0.5–2.5 kHz
in the frequency range 0.5–5 kHz
not more than 10 dB
not more than 20 dB
Noise level in the frequency range 500–5000 Hz not more than 30 dB SPL
DPOAE Technique
Frequency range 0.5–12 kHz
Stimulus intensity range 30–75 dB SPL
Stimulus 3rd order intermodulation not more than -80 dB
General Parameters and Specifications
Automatic result analysis yes
Indication of probe setting quality yes
Number of measurements saved in a device memory:
TEOAE
DPOAE
ABR
from 320 up to 1000
from 260 up to 700
from 1300 up to 4000
Number of patient cards saved in device memory:
TEOAE
DPOAE
ABR
from 320 up to 500
from 260 up to 350
from 1300 up to 2000
Operating time of electronic unit at rechargeable battery use from 7 up to 10* hours
LCD 3.5" with resolution not less
than 640480

Hearing Screening Systems (Technical Manual)
12
Table 1. Continued
Parameters Values
Interface Bluetooth
Electronic unit power supply voltage from external power supply
unit
9 V
Safety BF
Protection class from electrical shock 1
Electronic unit dimensions (19510155)2 мм
Electronic unit weight not more than 0.68 kg
Total weight not more than 2 kg
Power supply:
rechargeable battery
power supply unit:
model
supply voltage
output voltage
Li-ion with 4400 mAh
capacity
PSU-9
100–240 V, 50/60 Hz, 0.8 A
9 V DC
Note:
* depending on the operation mode of the device.
Safety and Electromagnetic Compatibility
Electromagnetic compatibility (EMC) is provided by IEC 60601-1-2:2007 requirements
fulfillment.
The screening systems are intended for operation in the electromagnetic environment
conditions which are specified in the appendix 1 “Electromagnetic Emission and
Immunity”.
Portable and mobile RF communication equipment may affect the system work.
The use of the equipment not listed in table 2 and table 3 of the present technical
manual may result in increased emission and system decreased immunity.
As for safety, screening systems satisfy IEC 60601-1:1988 + A1:1991 + A2:1995, IEC
60601-1-1:2000, IEC 60601-2-40:1998 standards requirements. The devices are
supplied by the external power supply unit of 9 V direct voltage or Li-ion rechargeable
battery, are related to class I and have BF type work parts according to IEC 60601-1.
All the computer equipment used together with the devices should correspond to IEC
60950-1 and CISPR 22:2006.
Interpretation of symbols on the electronic unit:
- attention: consult user and technical manuals.
- work parts of BF type according to IEC 60601-1.

Description and Operation
13
- mark of conformance to Russian standards requirements.
- mark of conformance to 93/42/EEC “Concerning Medical Devices”
directive.
- mark of conformance to 2002/96/EC “On waste electrical and electronic
equipment (WEEE)” directive.
Hearing Screening Systems (Technical Manual)
14
2.3. Arrangement and Operation
The screening system is intended for the performing of two test types: OAE and ABR.
The principle of operation at OAE performing is based on the registration of the
auditory fluctuations of the cochlea OHC with the use of the probe microphone, in
response to the auditory stimulation with the use of the telephones built in the probe.
The principle of operation at ABR performing is based on the registration of the brain
electrical response in reply to the auditory stimulation.
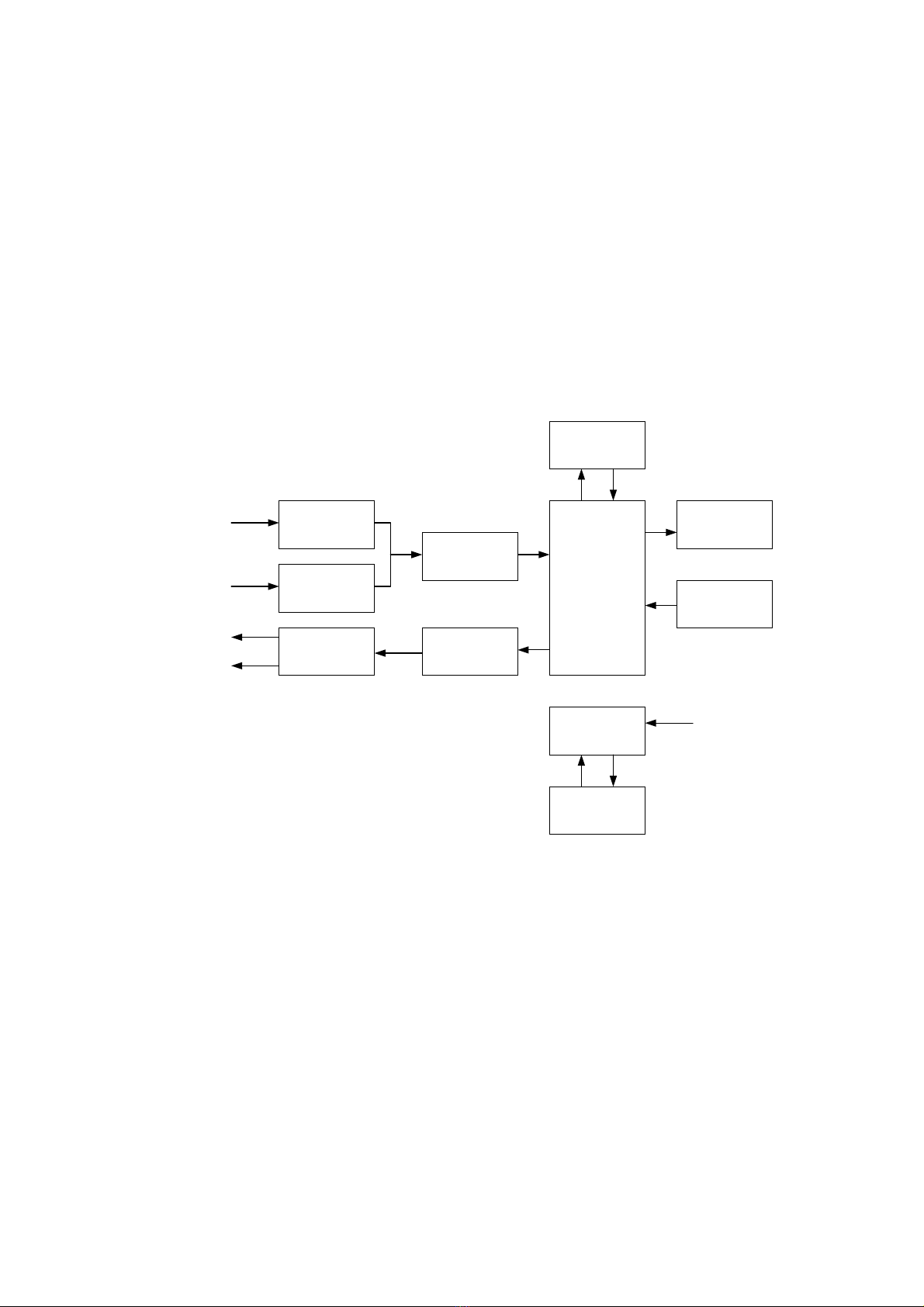
The functional scheme of Neuro-Audio-Screen screening system is presented in
the Fig. 1.
PU amplifier
Microphone
amplifier
Telephone
amplifier
ADC
DAC
CPM
LCD
Keyboard
Bluetooth module
Secondary power
supply source
Rechargeable
batteries
Biopotentials
electrodes
Probe microphone
Probe telephones
Headphones
External power
supply +9 V
Fig. 1
In the OAE test mode, the central processor module (CPM) generates the test signal
in a digital form and transfers it to the digital-to-analogue converter (DAC). The DAC
converts the signal to the analogue form and directs to the amplifier. After that, the
signal from the amplifier is transferred to the OAE probe telephones where it is
converted to the acoustic stimulus. The acoustic response of the cochlea OHC is
registered by the probe microphone and is converted to the electrical signal. Via the
microphone amplifier this signal is transferred to the analogue-to-digital converter
(ADC) input where it is converted to the digital form. The digitized response is directed
to CPM where it is processed and analyzed. The report concerning the test results is
based on the CPM analysis.
In the ABR test mode, the device operates in the same way. CPM generates the test
signal which is converted into the analogue form when transferring via the DAC, is
amplified by the amplifier and is directed either to the OAE probe telephones or to the

Description and Operation
15
headphones depending on what is used as an auditory stimulator. The brain electrical
response is recorded from the electrodes, directed to the biopotentials amplifier input
and then amplified. After that, it is transferred to the ADC, converted to the digital form
and directed to CPM. CPM processes and analyzes the received response and gen-
erates the report concerning the test results.
Except the above-mentioned functions, CPM controls completely the device
operation, keeps the settings and test results, displays the test procedure, test results,
service information using the LCD display. To transfer the results to the computer or
thermal printer and also to download the list of patients from the computer, CPM uses
Bluetooth module which provides the implementation of the Bluetooth wireless
interface. CPM is controlled by a user from a keyboard.
To provide the required supplying voltages for all the scheme units, the secondary
power supply source is provided. It can operate both from the built-in rechargeable
battery and external power supply. In the last case it provides the charge of the built-in
rechargeable battery.

Hearing Screening Systems (Technical Manual)
16
3. Description and Delivery Set
Neuro-Audio-Screen and Neuro-Audio-Screen/AOE screening systems are pocket
devices developed for the performing of the objective test of the auditory function. It
consists of the portable unit to which the OAE probe and electrodes for ABR recording
can be connected, the software for the data downloading from the device to the PC,
wireless thermal printer with Bluetooth interface for the printing of the tests results (is
not included in the base delivery set), set of ear tips and other accessories.
The delivery sets of Neuro-Audio-Screen and Neuro-Audio-Screen/AOE screening
systems are presented in the Table 2 and Table 3 (1 and 2 columns correspondingly).
Table 2. Base Delivery Set
Number, pcs.
Name Document code or main
specifications 1 2
Neuro-Audio-Screen electronic unit NSFT 057201.015 1 –
Neuro-Audio-Screen/OAE electronic
unit NSFT 057201.016 – 1
Power supply unit PSU-9 NSFT 057201.009 1 1
Power cord SCZ-1 (1.5 m.) 1 1
Bluetooth adapter1) 100 m, class 1, V.2.1, CSR chipset 4 4
Accessories for EP and OAE Studies
Cable for reusable electrodes
connection NSFT 057103.003-05 1 –
OAE probe 2) NSFT 006355.003-00
OAE-02-2
NSFT 006355.003-01
OAE-02-2
1 1
Set of OAE probe tips ER10D-RPT
NSFT 006221.001 1 1
Set of ear tips (pediatric) NSFT 007998.001 SP 1 1
Cable for disposable electrode
connection:
Alligator clip – touch-proof
(green, red or black)
NSFT 990103.027-02.05
NSFT 990103.027-03.05
NSFT 990103.027-04.05
1
1
1 –
Disposable surface electrode (100
pcs.) 1)
F3081, F3001 3
pack.
–
Test cavity NSFT 006201.013 1 1
Screw driver №1 GOST 17199 1 1
Dental floss Regular Oral-B (pack –
50 pcs)
Oral-B, Ireland 1 1
Probe tip extractor NSFT 006206.016 1 1

Description and Delivery Set
17
Table 2. Continued
Number, pcs.
Name Document code or
main specifications 1 2
Operational Documentation
Neuro-Audio-Screen,
Neuro-Audio-Screen/OAE technical manual TM057.02.002.000 1 1
Neuro-Audio-Screen, Neuro-Audio-
Screen/OAE calibration guidelines CG057.01.001.000 1 1
OAE probe guidelines GL032.03.001.000 1 1
Video manual on CD Version not lower than
001 1 1
Software
Neuro-Audio-Screen manager software without additional
modules 1 1
Package
Transportation bag - 1 1
Note:
1) The accessories and consumables of analogous types can be applied, but their use should
be permitted in the country.
2) OAE probe NSFT 006355.003-01 is supplied with probe tip NSFT 006221.001, and OAE
probe NSFT 006355.003-00 is supplied with probe tip ER10D-RPT.
Table 3. Additional Equipment, Accessories and Software
Number, pcs.
Name Document code or main specifications
1 2
Accessories for EP and OAE Studies
Auditory stimulator
(headphones)
NSFT 032305.005 (TDH-39)
NSFT 015305.001 (TA-01)
1 –
Insert earphones for
audiometry
ER3-10 1 –
Set of disposable foam ear tips
ER3-14A, ER3-14B, ER3-14D2, ER3-14C,
ER3-14E2
1 –
Adapter for earphones for
audiometry
NSFT 032103.004 1 –
NSFT 990106.028-02.05 (F8909Z)
NSFT 990106.027-02.05 (EEP) 1 –
NSFT 990106.028-03.05 (F8909Z)
NSFT 990106.027-03.05 (EEP) 1 –
Cup EP electrode with cable
(green, red, black) 1)
NSFT 990106.028-04.05 (F8909Z)
NSFT 990106.027-04.05 (EEP) 1 –

Hearing Screening Systems (Technical Manual)
18
Table 3. Continued
Number, pcs.
Name Document code
or main specifications 1 2
Consumables
Electrode adhesive paste 1)
TC 9398-011-34616468-2002
Unipasta, 120 g 1 –
Electrode abrasive paste
for skin preparation 1) Everi, Italy, 160 g. 1 –
Software
Neuro-Audio.NET
software
version not less than 1.0.6.0 1 1
Computer and Electronic Equipment 2)
System unit3) TC 4013-003-13218158-2011
“Functional”
“Elegant”
“Elite”
1 1
Notebook PC Minimal requirements
according to recommendations
stated in section 7.2
1 1
Monitor LCD 19’’ 1 1
Printer for personal
computer
Laser or jet 1 1
Printer for electronic unit CUSTOM S’Print BT
Citizen CMP10 2 Bluetooth
1 1
Note:
1) The accessories and consumables of analogous types can be applied, but their use should
be permitted in the country.
2) All the computer equipment should conform to IEC 60950-1 and CISPR 22 for B class.
3) The supply of other computer with the same or better specifications is allowed (see section
7.2).
The appearance of Neuro-Audio-Screen screening system with all the accessories
included in the base delivery set is shown in the Fig. 2.
Fig. 2. The appearance of Neuro-Audio-Screen screening system and accessories.
Mounting and Setting
19
4. Mounting and Setting
4.1. Unpacking and Check of Delivery Set
If the box with screening system was under conditions of the excessive moisture or
low temperature which differs sharply from working conditions, it is necessary to place
it in the room and leave for 24 hours in normal conditions.
Unpack the box, extract the screening system and the components. The delivery set
should correspond to the report concerning the device packing.
The computer equipment packed in the separate boxes should be opened according
to user and technical manuals for these products.
Check screening system and components to make sure that there are no external
damages.
4.2. Room Selection and Placement
The Neuro-Audio-Screen and Neuro-Audio-Screen/OAE screening systems are
portable devices that is why its exploitation is allowed in any rooms of patient care
institutions where the temperature and the humidity of the environment corresponds to
the conditions described in the device specifications. Also it is allowed to use the
device for the exam performing at patient’s place.
The room for OAE studies performing should be free from the noise
sources such as electrical motors, powerful ventilators, electric kettles,
audio equipment, aquarian compressors, etc.
4.3. Requirements to the Personnel Conducting
Mounting and Setting
There are no special requirements to the personnel conducting screening system
mounting and setting.
The replacement of the rechargeable battery must be performed in the service centers
of Neurosoft Company.

Hearing Screening Systems (Technical Manual)
20
4.4. Getting Started
The offered sequence of operations will allow you to speed up the use of
Neuro-Audio-Screen screening system for OAE recording. The operating order of
ABR test performing is described in section 5.8 “Patient Preparation for ABR Test”
and section 5.9 “ABR Test”. The first four steps are fulfilled in any case regardless of
the used technique.
First of all perform the otoscopic study prior to testing. Read carefully this technical
manual before you start to examine the patients.
Step 1. Connect the power supply unit to charge the battery. The battery is charged
for 3-4 hours (see section 5.3 “Rechargeable Battery Charge”).
Step 2. Connect the OAE probe to the screening system. Place the ear tip as far
down as possible on the OAE probe tip.
Step 3. Switch on Neuro-Audio-Screen by pressing on/off button for 2 seconds.
Step 4. After screening system loading, set the printer type and address if you have
wireless thermal printer. Enter the OAE probe sensitivity, date and time. Set the report
type. The detailed procedure is described in section 6.5 “System Settings”.
Step 5. Insert the ear tip deeply into the patient’s ear canal to obtain a seal. The order
of OAE probe insertion into the ear canal is described in section 5.5 “Patient Prepara-
tion for OAE Test”. Choose the technique and the tested ear with the use of “up/down”
buttons and “Select” key.
Step 6. First, Neuro-Audio-Screen checks the quality of OAE probe setting, performs
calibration automatically and then fulfills OAE recording. At high noise level the noise
level indicator highlights red. This is normal and it takes place quite often. Anyway the
study can be performed if the indicator does not glow red constantly, however it
impacts the final result of the recording. Once the test is finished, the test result is
displayed on the screen (PASS/REFER).
Step 7. After the test finishing, you can save the results in the screening system
memory by pressing “Close” button and answering “Yes” to the question “Save
exam?”. If you have printer, you can print the results. Switch on the printer by pressing
the round button on top. Press “Print” button on the screening system. The results of
the current exam will be printed.
This manual suits for next models
1
Table of contents
Other Neurosoft Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual














