serva Blue Series User manual

BlueLine
Instruments for Electrophoresis
INSTRUCTION MANUAL
___________________________________________________________________
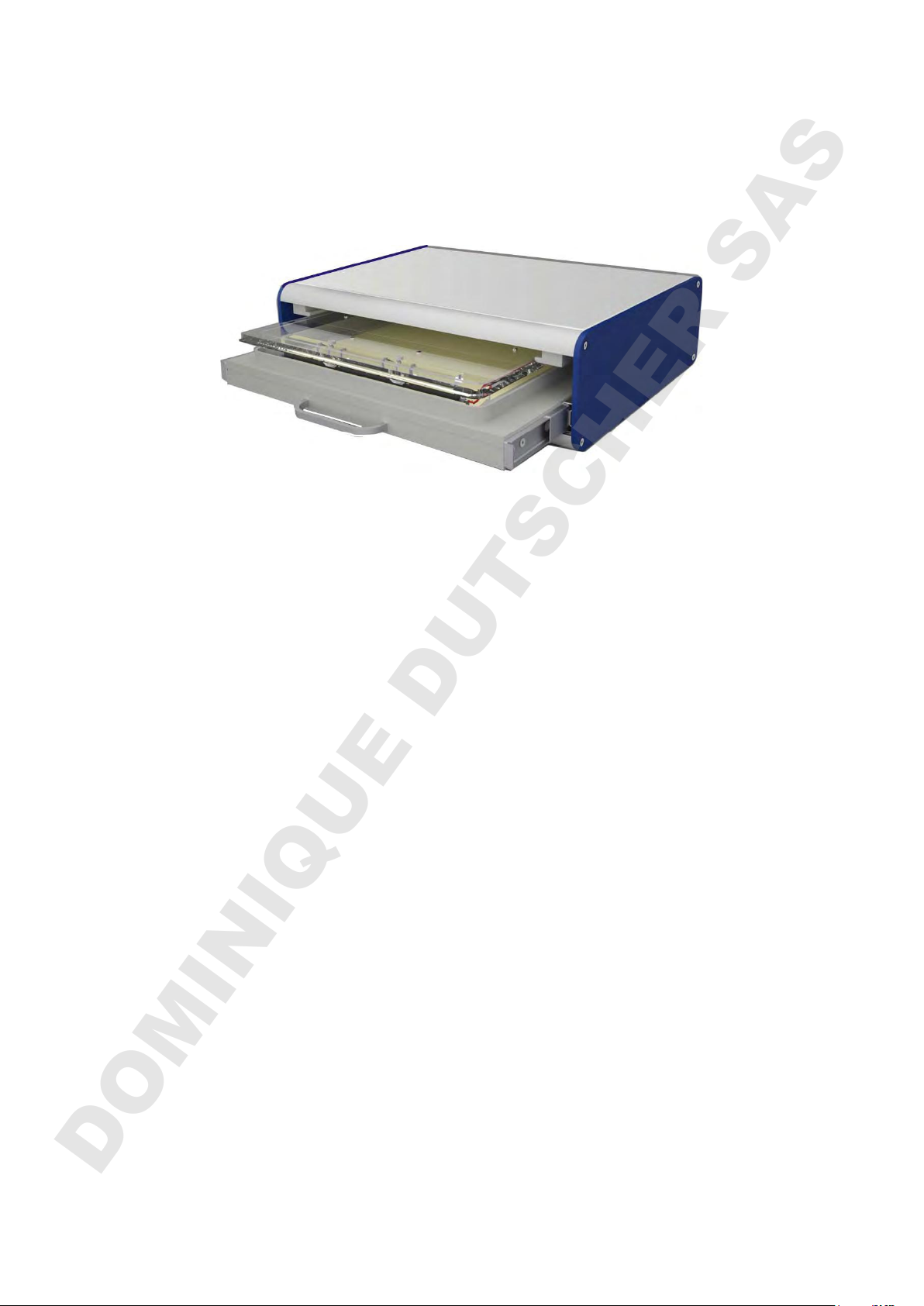
HPE™ BlueHorizon
Flatbed Electrophoresis System
(Cat. No.: HPE-BH)
SERVA Electrophoresis GmbH Carl-Benz-Str. 7 D-69115 Heidelberg
Phone +49-6221-138400, Fax +49-6221-1384010
e-mail: info@serva.de http://www.serva.de
DOMINIQUE DUTSCHER SAS

1
DOMINIQUE DUTSCHER SAS

2
Content
1 Safety ................................................................................................................... 3
2 Introduction .......................................................................................................... 4
3 Installation ............................................................................................................ 5
4 Operation ............................................................................................................. 6
4.1 Adjust the electrodes ..................................................................................... 6
4.2 Apply a gel ..................................................................................................... 6
4.3 Prepare Electrode Wicks ............................................................................... 7
4.3.1 SDS Gel Kit, CleanGel, HPE Gel ............................................................ 7
4.3.2 PreCotes, PreNets, CleanGel IEF ........................................................... 8
4.3.3 FocusGel................................................................................................. 8
4.4 Sample preparation and loading .................................................................... 8
4.4.1 SDS Gel Kit ............................................................................................. 8
4.4.2 CleanGel ................................................................................................. 8
4.4.3 HPE Gel .................................................................................................. 8
4.4.4 PreCotes, PreNets ................................................................................ 10
4.4.5 CleanGel IEF ........................................................................................ 10
4.4.6 FocusGel............................................................................................... 10
4.5 Start the run ................................................................................................. 11
4.6 After the run ................................................................................................. 12
5 Maintenance ...................................................................................................... 13
5.1 Cleaning ...................................................................................................... 13
6 Technical data .................................................................................................... 14
7 Explanations ...................................................................................................... 14
8 Trouble Shooting ................................................................................................ 15
9 Addendum: Example Applications ..................................................................... 19
10 Pack List and Order Information ........................................................................ 20
Vers. 01/14
DOMINIQUE DUTSCHER SAS
3
1 Safety
The SERVA HPETM BlueHorizon comply with the standards and directives mentioned
in the applicable CE declaration.
Please take note of the following safety measures:
Warning: Operation of this instrument requires high voltage.
Disconnect the high voltage external power supply before opening
any drawer.
Turn off and disconnect any high voltage power supply before
opening the safety lid.
Disconnect the high voltage external power supply and the AC
main supply before cleaning or servicing.
Do not spill or store liquids on top of the unit.
If liquid is spilled into the HPETM BlueHorizon, disconnect the high
voltage power supply and the AC main power immediately before
opening the safety enclosure lid.
Do not operate or connect power sources to the equipment if
there is any mechanical damage.
The supplied DC cables are rated for 5,000V. Only use cables
and adaptors supplied with the HPETM BlueHorizon or ensure that
these have a suitable DC insulation compliance for the used
voltages.
DOMINIQUE DUTSCHER SAS
4
2 Introduction
The HPETM BlueHorizon is a flatbed system for horizontal electrophoresis. Main
applications are isoelectric focusing (IEF), 2D PAGE, SDS PAGE and the separation
of nucleic acids in polyacrylamide gels.
The stable and easy-to-clean metal housing allows a space-saving positioning of the
power supply on top. The system is cost-saving, because it works without buffer
chambers. Instead, fabric wicks are soaked with concentrated electrophoresis
buffers.
The integrated drawer holds the cooling plate that is connected to the SERVA HPE™
Cooling Unit (cat. no. HPE-CU1). The cooling plate is made from a special ceramic
material (maximum gel size 260 x 205 mm) for efficient heat conductance down to 4
°C resulting in rapid and straight migration and therefore highly focused spots and
bands.
The electrode lid comes with one pair of platinum electrodes. They can be installed to
three electrode positions serving a wide variety of different gel sizes. A lid with triple
electrodes for bi-directional electrophoresis is available optional.
With the drawer / lid arrangement, the plastic-backed gels are protected from dust
and light during the run to avoid photo-bleaching of fluorescent labels.
DOMINIQUE DUTSCHER SAS
5
3 Installation
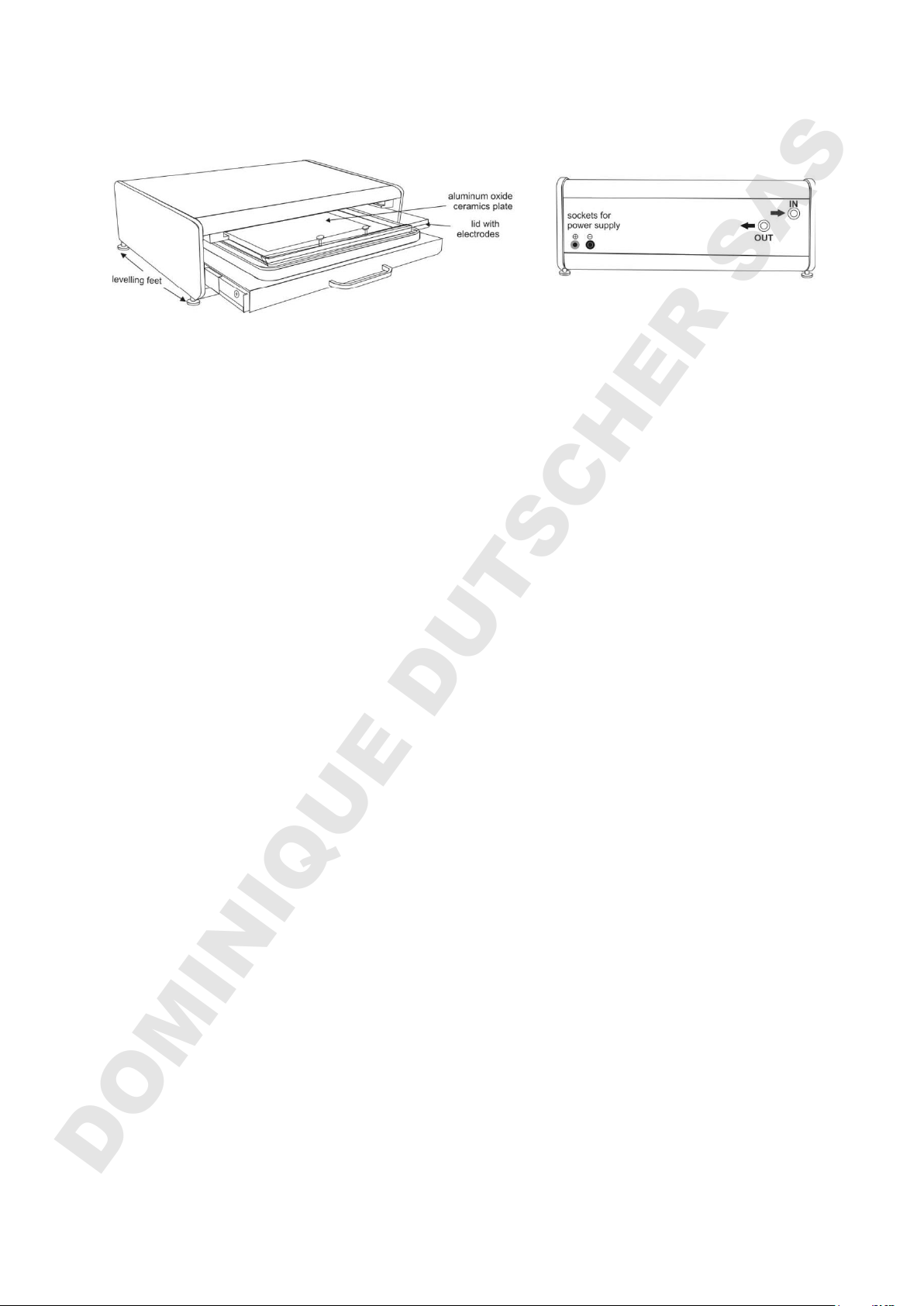
HPETM BlueHorizon (left: side/front view, right: back view)
The base-unit
Place the base unit on the bench. Unevenness of the lab bench can be corrected by
using a spirit level and adjusting the levelling feet.
Connecting the SERVA HPE™ Cooling Unit (Chiller)
Important note: Warm air should not be exhausted towards the BlueHorizon!
Connect the BlueHorizon to the chiller using the provided tubing and fix them with the
hose clamps. It is important to tighten these clamps sufficiently to obtain an air-tight
seal. The chiller ”Outlet“ must be connected to the BlueHorizon “Inlet” and the chiller
“Inlet” to the BlueHorizon “Outlet”. If the flow-direction is wrong, the cooling will work
incorrectly. In order to protect the cooling plate from corrosion, the cooling liquid must
contain an anti-corrosive additive (Cat. no. 43392)
Air Removal
For efficient cooling, it is vital to remove any air from within the cooling plate. Switch
on and leave the chiller running until all of the air in the connecting pipes is removed,
this may take several minutes. Then refill the chiller reservoir with cooling liquid (3
parts of water and 1 part anti-corrosive additive) as the BlueHorizon takes up about 1
litre.
Electrode Lid Storage
Remove the protection film and paper. Insert the lid into its “park” position” above the
drawer with the connecting plugs of the lid towards the backside of the BlueHorizon.
The electrodes are carefully constructed from platinum coated titanium rods and
could be damaged if not handled correctly. When not in use, always insert the lid into
its “park” position. Never place the lid on the bench with electrode side down to avoid
damage.
Connecting the Power Supply
Connect the power supply (black / red cables). Place it on top to save bench space.
DOMINIQUE DUTSCHER SAS
6
4 Operation
In this section, general instructions for loading and running gels on the HPETM
BlueHorizon are described. Running conditions for specific gel types, videos, and
other useful information are provided on the enclosed DVD and on www.serva.de.
Important Information: Always wear powder free disposable gloves when handling
gels or stripes. Do not open a drawer during an active electrophoresis run without
switching off or pause the power supply. Opening a drawer during running power
causes your power supply to detect a “ground leakage”. That may cause a
disturbance of the running programme or in the worst case damages the power
supply.
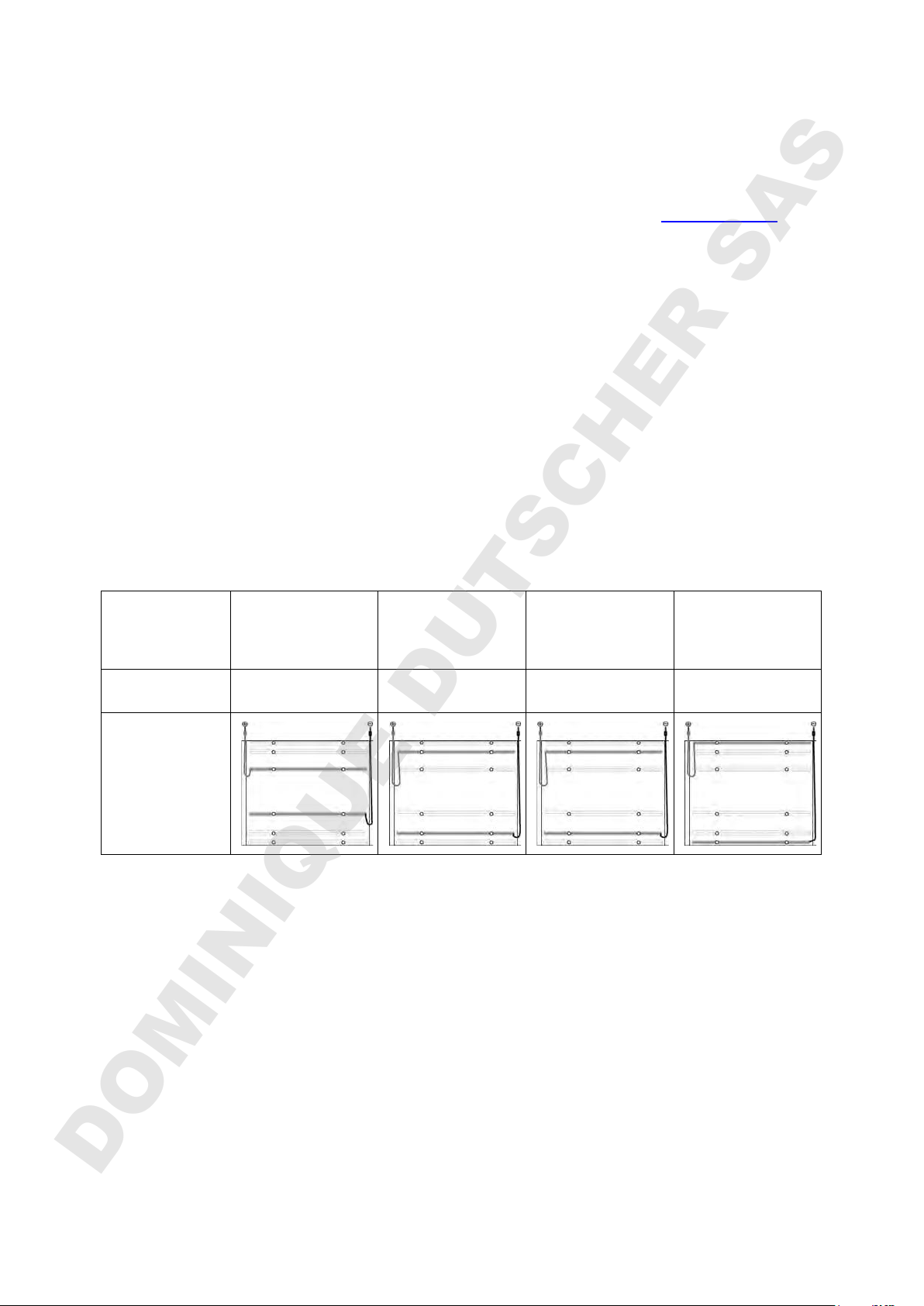
4.1 Adjust the electrodes
In the electrode lid, the electrode positions can be adjusted to different gels sizes.
When changing the electrode positions, place the electrode lid half way in the park
position and loosen the screws of the first electrode. Be careful to catch the nut on
the underside of the lid into which the screw fits. Then turn the lid and change the
second electrode as described above. Please note: The position for PreCotes is not
fixed but adjustable. Before each run with PreCotes or CleanGels IEF, superpose the
electrodes and the wicks on the gel.
Gels
PreCotes
CleanGel IEF
FocusGel
SDS Gel
CleanGel
2D HPE Triple /
Double Gels
2D HPE Large
Gel
Application IEF 1D PAGE 2D PAGE HiRes
2D PAGE
Electrode
position
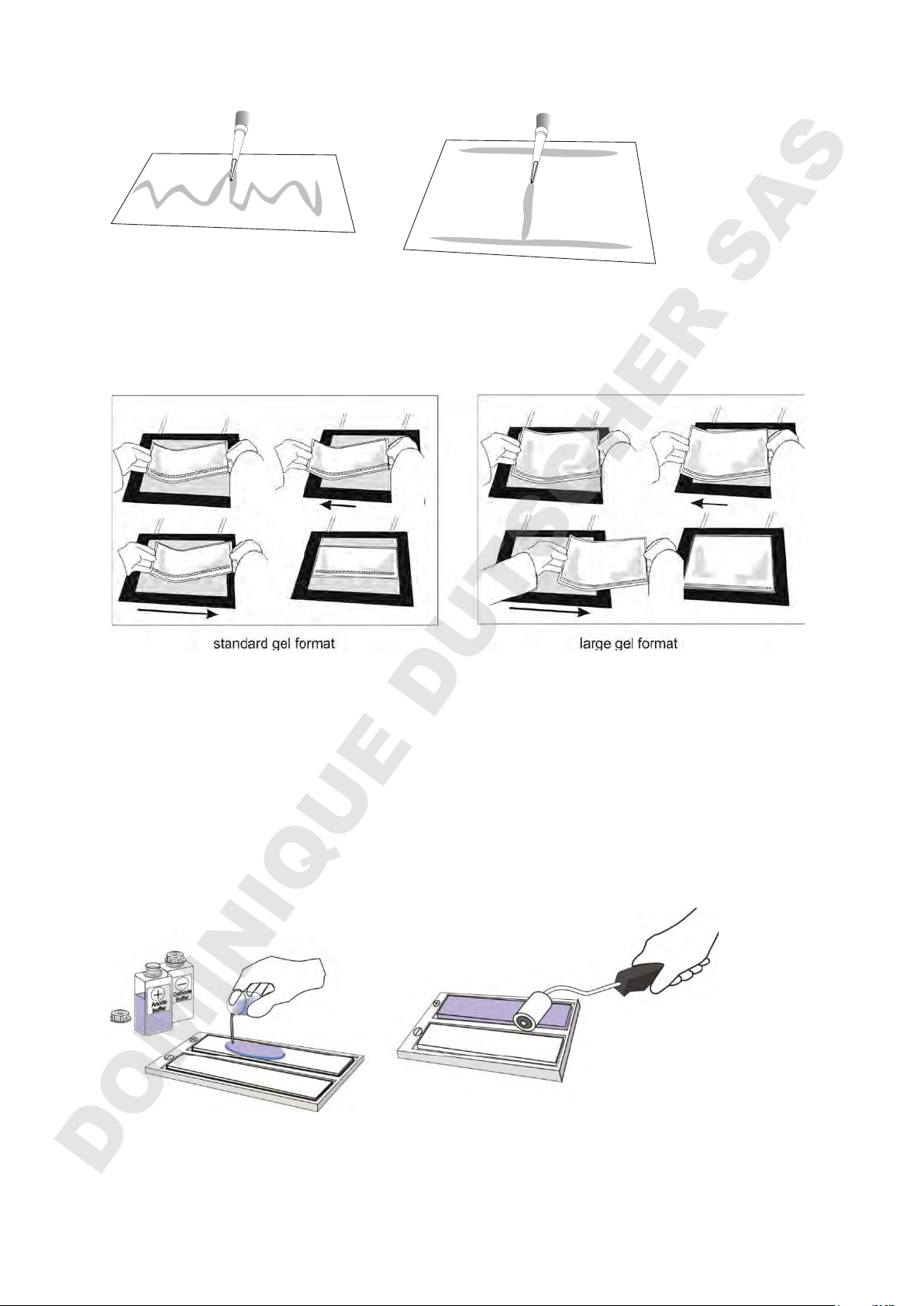
4.2 Apply a gel
Important Information: To avoid water condensation on the gel surface, do not yet
switch on the chiller.
A specially formulated cooling fluid is added between the surface of the cooling plate
and the gel to ensure good contact, even temperature control and efficient heat
dissipation. For a standard format gel spread 3 ml cooling contact fluid onto the
centre of the cooling plate. For a large format gel spread 6 ml cooling contact fluid
onto the cooling plate in the shape of an “H”:
DOMINIQUE DUTSCHER SAS
7
standard gel format large gel format
To disperse, slide a gel bent into a U-shape from side to side. The sides of the gel
are then gently lowered. Avoid air bubbles between the cooling plate and gel. Excess
cooling fluid from around the gel is removed using a lint-free tissue.
4.3 Prepare Electrode Wicks
4.3.1 SDS Gel Kit, CleanGel, HPE Gel
All buffers needed are provided in the SERVA Gel and Buffer Kits. We do not
recommend different or self-made components.
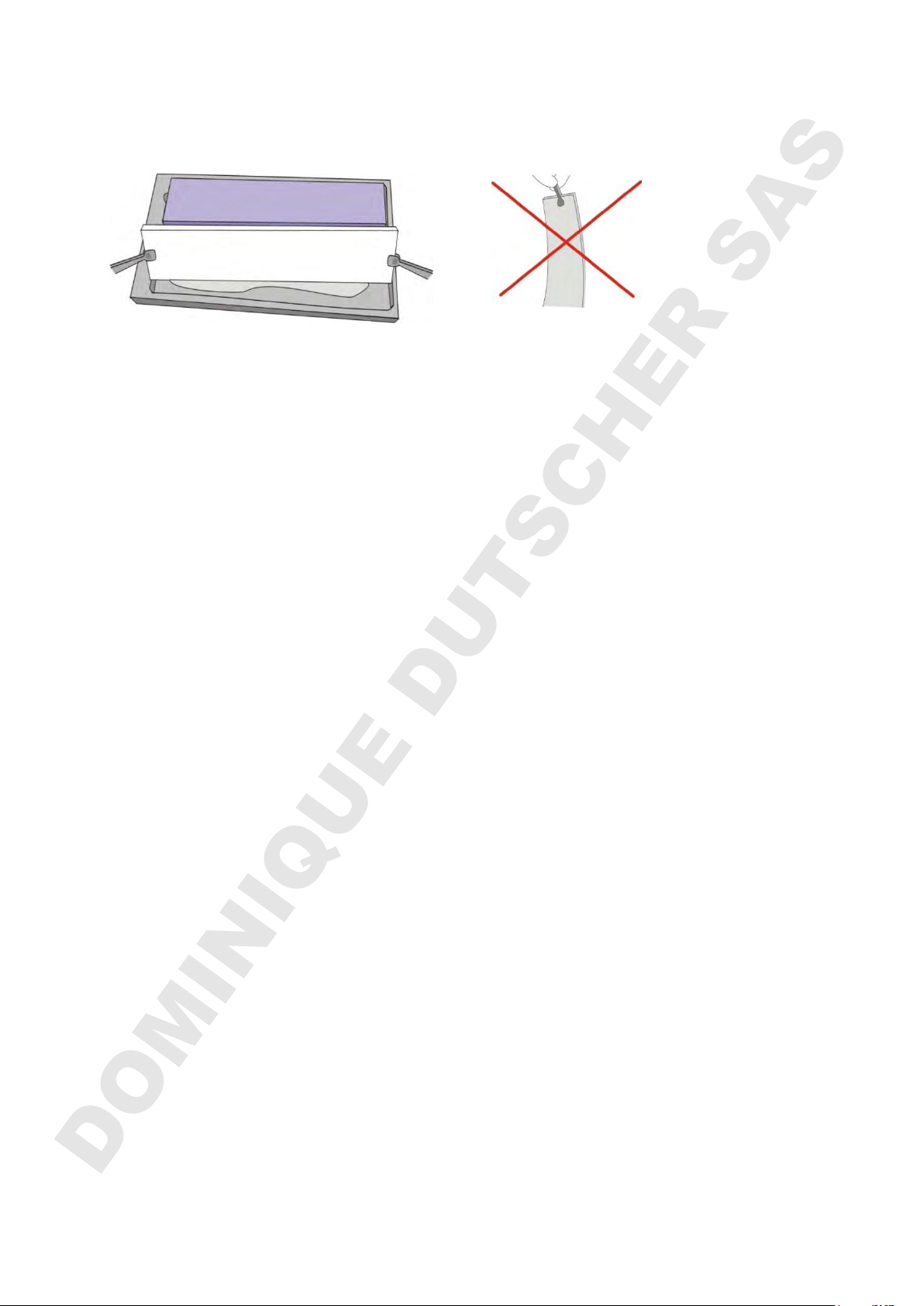
Electrode wicks, soaked in an appropriate buffer, provide a convenient alternative to
buffer tanks. The wicks should be fully soaked with 45 ml buffer for at least 10
minutes. The wicks should be rolled to remove air bubbles and to distribute the buffer
evenly using the supplied roller:
Electrode wicks are applied with the cathode (white) at the front, anode (blue) at the
back. Remove excess electrode buffer from the wicks by tilting the electrode wicks
along one long edge and dab it on the paper pool bottom. When moving the wicks
DOMINIQUE DUTSCHER SAS
8
always hold them horizontal, as holding them at a vertical angle can result in unequal
buffer concentration.
The electrode wicks should overlap the gel by at least 2 mm. It is important that
buffer is not dropped onto the gel surface and therefore avoid moving the buffer
soaked wicks over the gel.
4.3.2 PreCotes, PreNets, CleanGel IEF
Place two electrode wicks (5 mm) soaked with anode and cathode buffer. Apply them
on the corresponding gel edges: acidic solution on the Anode (+) side, basic solution
on the cathode (-) side. Wicks must not extend beyond edge of gel but be aligned
parallel to each other and corresponding to where the electrodes will be placed.
4.3.3 FocusGel
On FocusGels, the electrodes are directly placed onto the gel without the need of
buffer soaked wicks.
4.4 Sample preparation and loading
4.4.1 SDS Gel Kit
1) Add one volume sample to one volume sample buffer (2x) and dilute the sample
to loading concentration with the sample diluter (depends on the sensitivity of
staining method). Reduce and alkylate your sample.
2) Pipette samples into the sample wells.
4.4.2 CleanGel
Prepare samples according to the related gel instruction manual or specific
application procedure.
4.4.3 HPE Gel
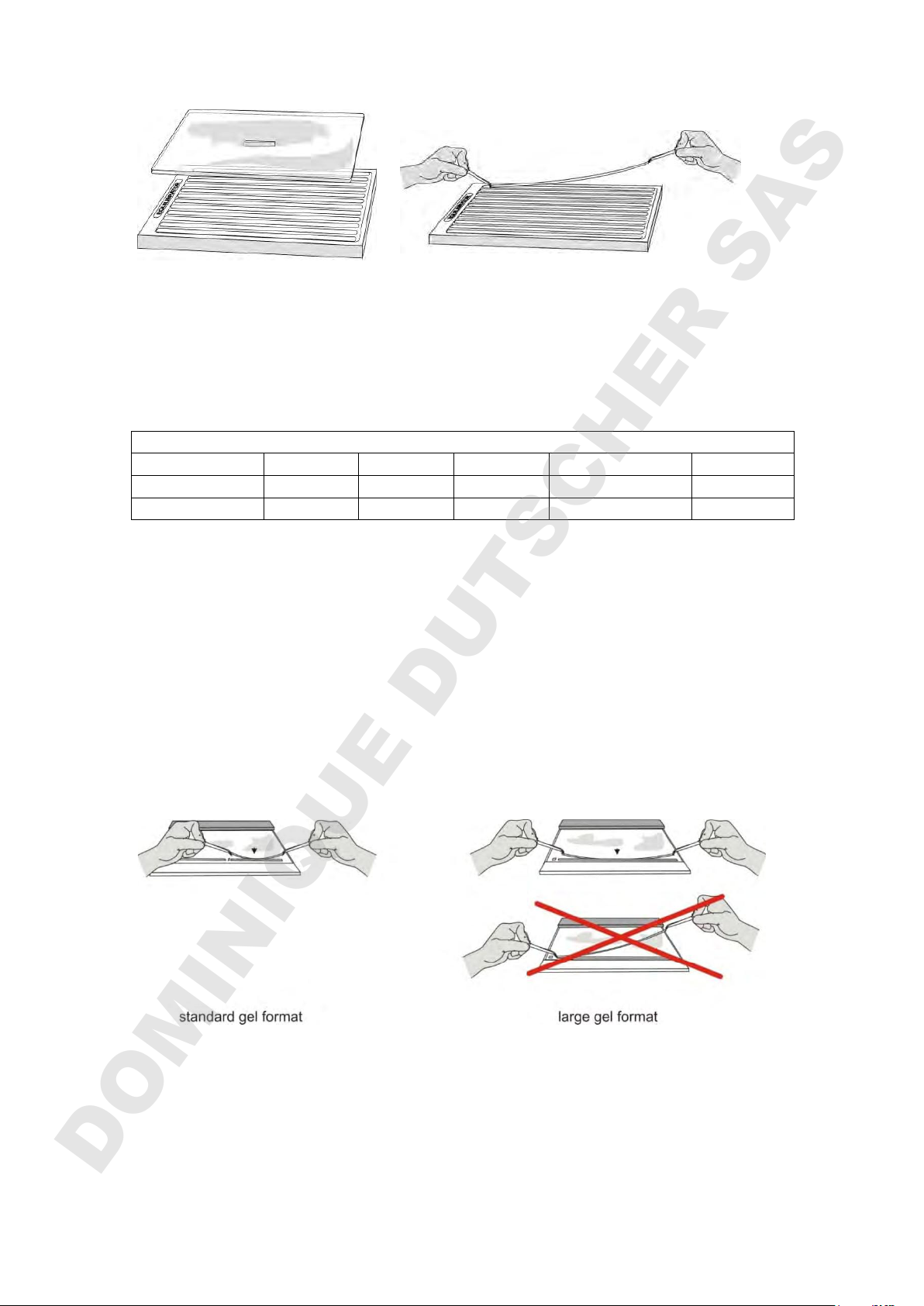
1) Equilibrate the IPG strip
SERVA IPG strip equilibrator (Cat. no. HPE-A04) provides a convenient way to
equilibrate IPG strips. After equilibration, the strip can be easily transferred from
the slots holding the first equilibration solution (e.g. DTT) to the slots holding the
second solution (e.g. IAA).
DOMINIQUE DUTSCHER SAS
9
Prepare the two equilibration solutions from the SERVA IPG Strip equilibration
buffer:
DTT solution: Weigh urea and Dithiothreitol (DTT), add the equilibration buffer
and dissolve completely.
IAA solution: Weigh urea and Iodoacetamide (IAA), add the equilibration buffer
and dissolve completely.
Sufficient amount for 1 x 18cm, 1 x 24 cm, 2 x 11cm, 3 x 7cm
Urea [g] DTT [mg] IAA [mg] Eq. Buffer [ml] Total [ml]
DTT solution
1.8
50
-
5
6
IAA solution 1.8 - 125 5 6
Equilibrate each strip in 6 ml (18 or 24 cm strips), 3 ml (11 cm strips) or 2 ml (7
cm strips) solution in an equilibrator on an orbital shaker with 30 –50 /min in DTT
for 15 min and in IAA solution for 15 min.
2) Applying the IPG-strip
IPG strips should still contain equilibration buffer on their surfaces. Do not blot
IPG strips dry, this can cause vertical streaking in the second dimension due to
insufficient protein transfer. Some IPG strips have rather long protruding support
film on the ends: In this case the plastic film support on both sides of the IPG-strip
must be trimmed just beyond the gel.
The strip should be carried horizontally and applied to the slot center first.
The strip should be placed in the IPG slot, gel side down, with the anodal side to
the right. To ensure good contact in the slot the back of the forceps is slid gently
along the back of the IPG strip.
DOMINIQUE DUTSCHER SAS

10
4.4.4 PreCotes, PreNets
1) Unpack the gels and remove the protective cover-film from the gel surface. Keep
the cover film as it can serve as a protective sheet later.
2) Adjust sample concentration to about 1 - 10 mg protein/ml and desalt by dialysis;
by dilution with dest. water or by lyophilization and resuspension in dest. water.
3) Centrifuge the samples for 5 minutes at approx. 12,000 g; use only the
supernatant. By omitting this step the separation pattern might become fuzzy and,
eventually, precipitates may form within the applicator strip slots.
4) Position the applicator strip on the gel and slightly pressing it with the back of a
forceps. Apply the required sample volume using a pipette. Do not leave empty
slots between samples. Depending on sample type, it is possible to apply the
samples with or without pre-focusing.
4.4.5 CleanGel IEF
1) Unpack and rehydrate the dry gel according to the gel instruction manual.
2) Prepare and apply samples on the gel according to the related gel instruction
manual or specific application procedure.
4.4.6 FocusGel
1) Unpack the gels and remove the protective cover-film from the gel surface. If
necessary, remove excessive moisture from the gel surface with the edge of a
drying cardboard. Keep the cover film as it will serve as a protective sheet later.
The gel is ready to use.
FocusGel 24S and 40S: For some sample types, e.g. serum and CSF the position
of the pre-formed wells is optimized for anodal application in a pH gradient 6-11.
This well position might also be suitable for other sample types. Nonetheless, the
gels can be turned around for cathodal application if needed.
2) Apply the required sample volume using a pipette. Do not leave empty slots
between samples. Depending on sample type, it is possible to apply the samples
with or without pre-focusing.
3) Take care that the electrodes are placed directly on the FocusGel surface at the
gel edges and not on the support film!
DOMINIQUE DUTSCHER SAS
11
4.5 Start the run
1) Close the lid while lowering the electrodes on the wicks. Plug in the leads.
2) Switch the thermostatic circulator on, set to
HPE Gels: 15 °C
SDS Gels: 15 °C
Clean Gels: dependent on the application (see gel manual)
PreCotes, PreNets: 10°C
CleanGel IEF: 10°C
Focus Gel: dependent on the application (see gel manual)
Note: During Electrophoresis the electric resistance of the gel is slowly increasing.
Therefore, the heat production during the starting phase is rather low. It does not
cause overheating when the chiller begins cooling at the same time the
electrophoresis is started.
3) Start the run according to the settings described in the gel manual.
DOMINIQUE DUTSCHER SAS

12
4.6 After the run
After electrophoresis, clean the device as described and follow with staining and
detection. We recommend:
Staining
Application
Cat. No.
SERVA HPE™ Silver Staining Kit (MS compatible) 2D 43395
SERVA CSF Silver Staining Kit CSF IEF 43398
SERVA Silver Staining Kit Native PAGE Native PAGE 35077
SERVA Silver Staining Kit SDS PAGE SDS PAGE 35076
SERVA HPE™ Coomassie® Staining Kit PAGE, 2D 43396
SERVA Blue G PAGE 35050, 17524
SERVA Blue R PAGE 35051, 17525
SERVA Blue R Staining Kit PAGE 42531
SERVA DensiStain Blue G Staining Solution PAGE 35078
Quick Coomassie™ Stain IEF, PAGE GEN-QC-STAIN-1L
SERVA Blue W IEF 35053
SERVA Violet 17 Staining Kit IEF, native PAGE 35074
SERVA Violet 17 IEF, native PAGE 35072
SERVASnow Staining Kit PAGE background stain 35080
SERVA ProteinStain Fluo-R (MS compatible) IEF, PAGE, 2D 35091
SERVA Purple (MS compatible) PAGE, 2D 43386
SERVA Lightning Red for 1D SDS PAGE (label) PAGE 43401
SERVA HPE™ Lightning Red (label) 2D 43400
DOMINIQUE DUTSCHER SAS

13
5 Maintenance
5.1 Cleaning
Regularly clean the housing of the SERVA HPETM BlueHorizon.
Precautions for avoiding electric shock
Electronic devices can cause electric shocks in case of an operating
error. Never try to repair electric parts. Never open the housing.
Switch off the instrument and disconnect it from the power supply
before starting with cleaning or disinfection works.
Never let get liquids inside the housing.
Do not perform spray disinfection.
Do only connect the instrument with the power supply if it is
completely dry.
The repair service may only be performed by authorized staff trained by
the manufacturer. A modification of the instrument is not permitted.
Caution when handling aggressive chemical
s
Do not use aggressive chemicals e.g. strong and weak bases, strong
acids, formaldehyde, acetone, halogenated hydrocarbons, phenol and
other organic solvents for cleaning the instrument and its accessories.
In case of contamination with aggressive chemicals, clean the
instrument with a neutral detergent immediately.
Use neither corrosive detergents nor aggressive solvents or
abrasive polishing agents.
Cleaning
When running the BlueHorizon for the first time, or if the cooling plates became
soiled, clean them using a 0.1% SDS solution, followed by isopropyl alcohol and
finally distilled water. Disconnect the instrument from the power supply before you
start cleaning to obtain the best results it is recommended to clean the cooling plates
and electrodes before and immediately after use with distilled water. The cooling
plate can be gently rubbed and dried using a lint-free tissue. Abrasive cleaners and
other solvents must not be used. Cleaning of the platinum electrodes after each run
is particularly important to prevent crystallization of buffer salts, which can result in
uneven contact. Electrodes should be cleaned with distilled water-moistened lint-free
tissue.
DOMINIQUE DUTSCHER SAS
14
Disinfection
1. Disconnect the instrument from the power supply before you start disinfecting.
2. Let the instrument cool down.
3. Clean the instrument as described above.
4. Select a disinfection method compliant to the applicable local legal regulations
and directives.
5. Wipe off all outer parts of the instrument with the disinfectant and a lint-free
cloth.
Decontamination before shipment
If you need to send the instrument back to us, decontaminate all parts. Document this
in our Decontamination Certificate (Download on www.serva.de) and include it within
the shipment.
6 Technical data
SERVA Blue
Horizon
Max. Spannung, Strom / 3000V, 25mA
Max Voltage, Current
Max. Gelgröße / 260 x 205 mm
Max gel size
Temperatur-Arbeitsbereich / +4°C to +30°C
Temperature-working range
Elektrodenabstand / 270, 195, 115 mm
Electrode distance
Abmessungen (B x T x H) / 450 x 500 x 120 mm
Dimensions (W x D x H)
Gewicht (ohne Block) / 6 kg
Weight (without block)
7 Explanations
Attention! Electric shock!
Attention!
DOMINIQUE DUTSCHER SAS

15
8 Trouble Shooting
Symptom Cause Remedy
7.1 Effects during electrophoresis
No water flow after
chiller is switched
on.
Wrongly connected
tubing or kinked tube.
Straighten tubing. Check tubing:
Chiller-“Out“ connected to chamber-
“In“.
Air bubbles
between film-
backing and cooling
plate.
Insufficient volume of
cool contact fluid
Lift up gel on one side and apply a
higher volume
Excess cooling fluid
around the film
support.
Too much cooling fluid
applied on the cooling
plate.
Remove excess fluid with lint-free
tissue paper.
Water droplets on
gel surface.
Gel was pre-cooled
without lid at high
humidity conditions
leading to water
condensation.
Do not switch on chiller during gel
application and sample loading.
No electric current,
drawer control
lamps do not
illuminate after
starting the power
supply.
Electronic control
detects wrong
orientation of electric
field.
Plug power supply cables in correctly:
black cable to cathode, red cable to
anode.
Electronic control
detects that one or
more draws do not
contain a gel.
When less than four gels are run,
unplug the non-used electrode lids
and place them into the parking
position.
Lids not properly
positioned or not
plugged-in.
Re-position lids and check
connections between lids and
drawers.
Condensation
inside of electrode
lid.
The gel gets hot
during electrophoresis
because of insufficient
heat dissipation.
Do not forget to switch chiller on!
Check chiller temperature and ensure
no other apparatus is connected to
the same chiller.
DOMINIQUE DUTSCHER SAS

16
Front is curved
instead of straight.
The gel gets hot
during electrophoresis
because of insufficient
heat dissipation.
See above
BlueHorizon is subject
to hot exhaust from
chiller or other
apparatus.
Relocate chiller or other apparatus.
The gel gets hot
during electrophoresis
because too much
power is applied per
gel.
If you run less than four gels at
a time,
reduce the mA and W settings in the
power supply accordingly. Follow
strictly the manual.
Migration of front is
very slow and will
not reach the anode
in time.
The electric field is too
low. Adjust the mA and W settings
according to the number of gels run.
Front is slanted, not
straight.
Uneven buffer
concentration within
the electrode wicks.
Always hold electrode wicks
horizontal when carrying them to the
gel.
Condensation water
develops inside
electrode lid near
to IPG strip(s).
Local heat production
at IPG strip(s)
because of
electroendosmotic
effect.
Remove IPG strip(s) from gel after the
first 70 minutes and then continue the
run. Follow strictly the manual.
Minor
disturbance(s) in
the front.
Buffer drop(s) fell on
the gel surface. Avoid passing wicks over gel surface.
Air bubbles inside the
wicks.
Gently roll wicks in PaperPool to
remove air.
2D Gels: Irregular
bulging of the front
on one side.
Equilibration buffer
unequally distributed
within the IPG strip(s).
Hold the IPG strip(s) horizontal, start
in the middle when placing the strip
into the slot.
DOMINIQUE DUTSCHER SAS

17
2D Gels: Run stops,
front does not
continue to migrate,
sparking at the IPG
strip(s).
Strong
electroendosmosis
effect at the IPG
strip(s), because it
has not been removed
after the first 70
minutes.
Remove IPG strip(s) from gel after the
first 70 minutes and then continue the
run. Follow strictly the manual.
7.2 Effects during scanning
Gel edges curl up
during scanning.
Gel edges start to dry
out.
Apply Scan-Frame on the edges of
the gel during scanning.
12.4 Effects seen in the result
2D Gels: Horizontal
streaking
The first dimension
IEF separation in the
IPG strip did not work
well because of
inappropriate sample
preparation IEF
separation problems.
Check the trouble shooting guides
supplied by the providers of the IPG
strips and web forums.
2D Gels: Vertical
streaking
Insufficient
equilibration of the
IPG strip.
Use the equilibration buffer supplied
with the HPE gels, weigh-out the
correct amounts of DTT and IAA
(should be of highest reagent quality),
follow the manual.
IPG strip has become
too dry.
Ensure that there is still a thin layer of
buffer on the surface.
Local disturbances
in the pattern
Air bubble in the
cooling contact fluid
layer
Use sufficient cooling contact fluid on
the cooling plate (3 for standard size
gels, or 6 ml for large gels), distribute
is evenly by sliding the gel with the
film-backing several times left and
right.
Air bubble in a buffer
wick
Distribute the buffer solutions evenly
in the wicks by thoroughly rolling
DOMINIQUE DUTSCHER SAS

18
SERVAPurple
staining:
Unsufficient
sensitivity of
staining
Wrong protocol (for
non-backed gels) has
been applied, or the
solution volumes were
too small
Follow the protocol of this HPE
Horizon manual which has been
optimized for the HPE Flatbed gels.
DOMINIQUE DUTSCHER SAS

19
9 Addendum: Example Applications
1D SDS PAGE 25 slots
SDS Gel Kit 10% 25S; Cat. No. 43359
Marker, muscle, E. coli extracts
Coomassie® staining
SERVA Blue R Staining Kit; Cat No. 42531
1D native PAGE 52 slots
CleanGel 10% 52S; Cat. No. 43340
Native Buffer; Cat. No. 43352
Tomato seeds
Coomassie® staining
SERVA Blue R Staining Kit; Cat No. 42531
Precast Horizontal Gel for Isoelectric Focusing
FocusGel 6-11 24 slots; Cat. No. 43329
Cerebrospinal fluids and serum
Ammoniacalic silver staining with SERVA CSF
Silver Staining Kit; Cat. No. 43398
2D Electrophoresis
2D HPE™ Triple-Gel NF 12.5 % Kit; Cat. No.
43300
E. coli extracts
ServaPurple™ staining; Cat. No. 43386
2D Electrophoresis
2D HPE™ Double Gel NF 12.5 % Kit, Cat. No.
43302
Human serum proteins
ServaPurple™ staining; Cat. No. 43386
2D Electrophoresis
2D HPE™ Large Gel 12.5 % Kit,Cat. No. 43310.
E.coli proteins
ServaPurple™ staining; Cat. No. 43386
DOMINIQUE DUTSCHER SAS
This manual suits for next models
2
Table of contents
Other serva Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual













