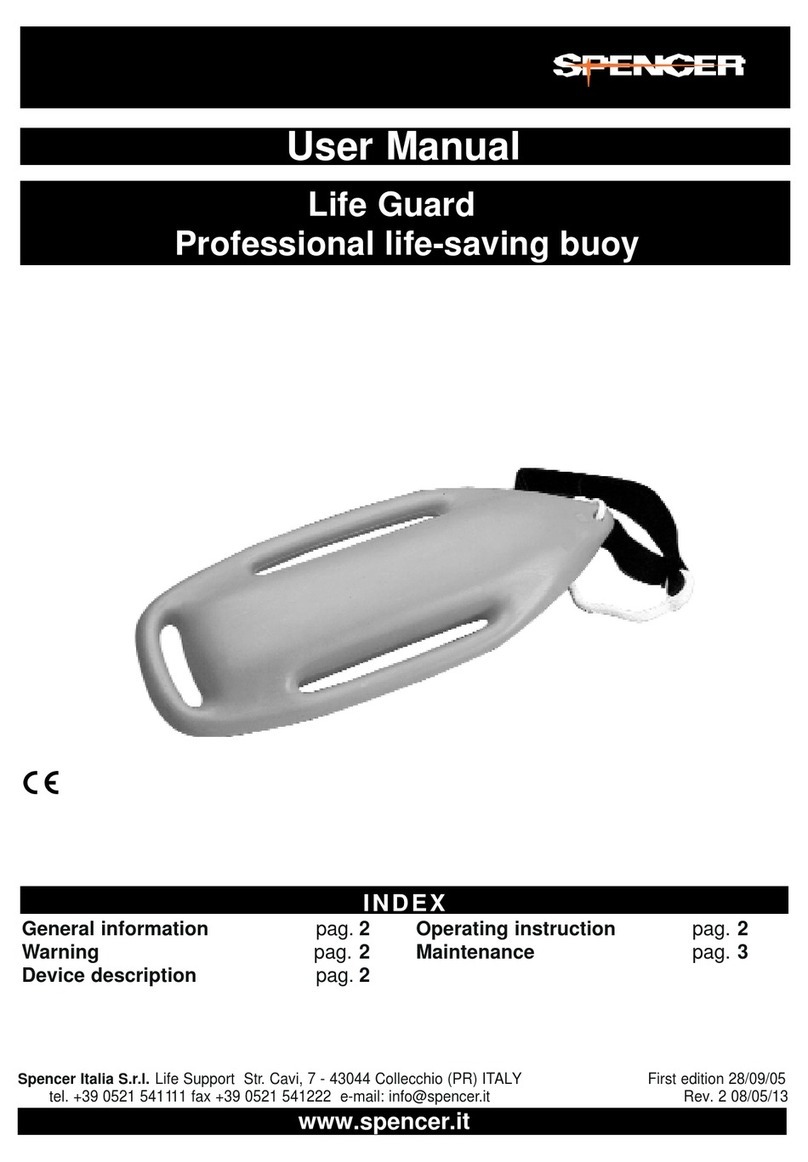
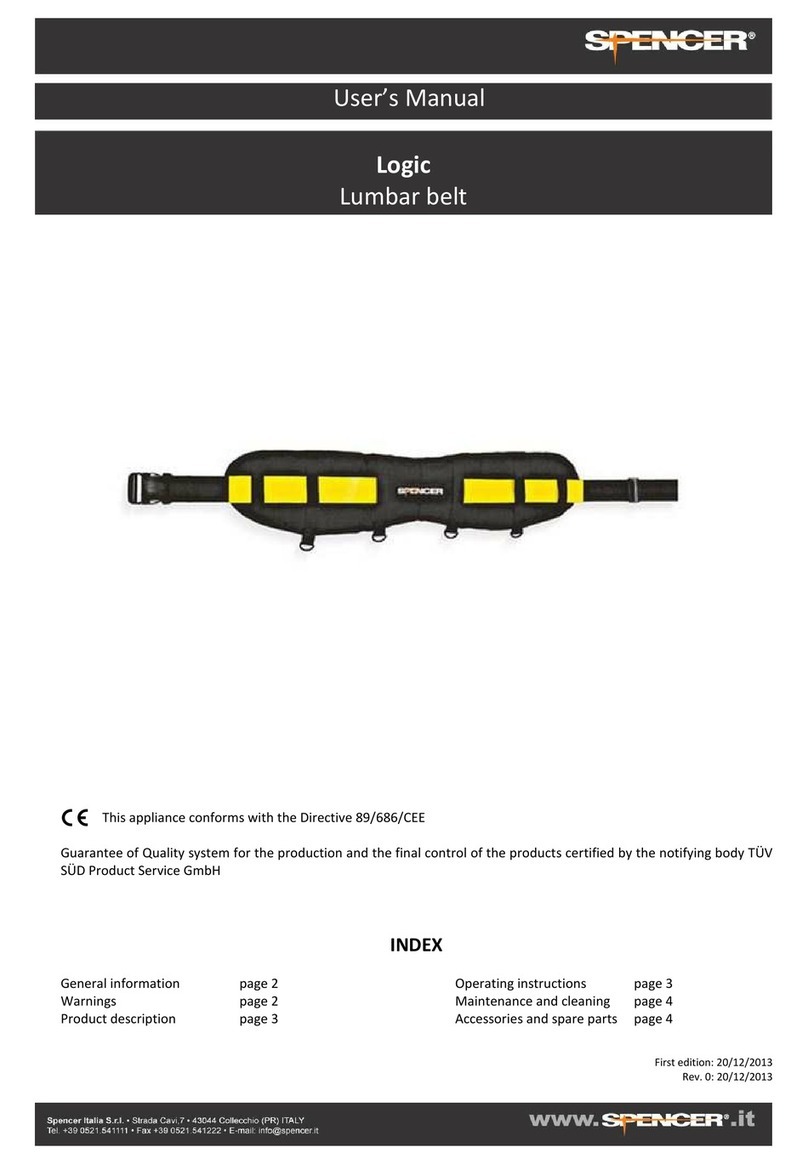
Spencer SKID-E User manual

SPENCER ITALIA SRLSPENCER ITALIA SRL – Via Provinciale n° 12
43038 Sala Baganza (PR) – Italy
IT
Manuale d’Uso e Manutenzione
SKID-E, PRO SKID-E, PRO SKID-E MAX, SKID-E READY,
SKID-OK, SKID-OK MAX
Sedie di evacuazione
DE
Bedienungs- und Wartungshandbuch
SKID-E, PRO SKID-E, PRO SKID-E MAX, SKID-E READY,
SKID-OK, SKID-OK MAX
Evakuierungsstühle
EN
User’s Manual
SKID-E, PRO SKID-E, PRO SKID-E MAX, SKID-E READY,
SKID-OK, SKID-OK MAX
Evacuation chairs
Sistema di Garanzia di Qualità per la produzione ed il controllo inale dei prodotti
certiicato dall’organismo notiicato TÜV SÜD Product Service GmbH.
Guarantee of Quality system for the production and the inal control of the products
certiied by the notifying body TÜV SÜD Product Service GmbH.
Qualitätssicherungssystem für die Herstellung und Endkontrolle von Produkten,
zertiiziert durch die benannte Stelle TÜV SÜD Product Service GmbH.
Dispositivo Medico
conforme al Regolamento
UE 2017/745
The device is in compliance
with the Regulation UE
2017/745
Es wird hiermit erklärt, dass
das Gerät der EU-Verordnung
2017/745 entspricht

2
INDICE / CONTENTS / INHALTSVERZEICHNIS
1. INFORMAZIONI GENERALI 4
1.1 SCOPO E CONTENUTO 4
1.2 CONSERVAZIONE DEL MANUALE D’USO 4
1.3 SIMBOLI 4
1.4 RICHIESTA DI ASSISTENZA 4
1.5 SMALTIMENTO 4
1.6 ETICHETTATURA 4
2. AVVERTENZE 4
2.1 AVVERTENZE GENERALI 4
2.2 AVVERTENZE SPECIFICHE 5
2.3 CONTROINDICAZIONI ED EFFETTI COLLATERALI 5
2.4 REQUISITI FISICI DEGLI OPERATORI 5
3. DESCRIZIONE DEL PRODOTTO 6
3.1 DESTINAZIONE D’USO 6
3.2 UTILIZZATORI PREVISTI 6
3.3 PAZIENTI ATTESI 6
3.4 COMPONENTI PRINCIPALI 6
3.5 MODELLI 7
3.6 DATI TECNICI 7
3.7 STANDARD DI RIFERIMENTO 7
3.8 CONDIZIONI AMBIENTALI 7
4. ISTRUZIONI OPERATIVE 7
4.1 TRASPORTO E STOCCAGGIO 7
4.2 PREPARAZIONE 7
4.3 FUNZIONAMENTO 8
4.3.1 APERTURA DISPOSITIVO 8
4.3.2 CHIUSURA DISPOSITIVO 8
5. TABELLA GESTIONE GUASTI 9
6. PULIZIA E MANUTENZIONE 9
6.2.1 MANUTENZIONE ORDINARIA 10
6.2.2 MANUTENZIONE STRAORDINARIA 10
6.2.3 REVISIONE PERIODICA 10
6.3 TEMPO DI VITA 10
7. ACCESSORI 10
8. RICAMBI 10
ALLEGATO A – REGISTRO DELLA FORMAZIONE 11
ALLEGATO B – REGISTRO DELLA MANUTENZIONE 12
1. GENERAL INFORMATION 13
1.1 AIM AND CONTENTS 13
1.2 CONSERVATION OF THE INSTRUCTION MANUAL 13
1.3 SYMBOLS USED 13
1.4 SERVICING REQUEST 13
1.5 DEMOLITION 13
1.6 LABELLING 13
2. WARNINGS 13
2.1 GENERAL WARNINGS 13
2.2 SPECIFIC WARNINGS 14
2.3 CONTRAINDICATIONS AND SIDE EFFECTS 14
2.4 PHYSICAL REQUIREMENTS OF THE OPERATORS 14
3. PRODUCT DESCRIPTION 15
3.1 INTENDED USE 15
3.2 INTENDED USERS 15
3.3 PATIENTS 15
3.4 MAIN COMPONENTS 15
3.5 MODELS 16
3.6 TECHNICAL DATA 16
3.7 REFERENCE STANDARDS 16
3.8 ENVIRONMENTAL CONDITIONS 16
4. OPERATING INSTRUCTIONS 16
4.1 TRANSPORT AND STORAGE 16
4.2 PREPARATION 16
4.3 FUNCTIONING 17
4.3.1 OPENING THE DEVICE 17
4.3.2 CLOSING THE DEVICE 17
DOWNSTAIRS 18
5. TROUBLESHOOTING 18
6. MAINTENANCE AND CLEANING 18
6.1 CLEANING 18
6.2 MAINTENANCE 18
7. ACCESSORIES 19
8. SPARE PARTS 19
ATTACHMENT A – TRAINING REGISTER 20
ATTACHMENT B – MAINTENANCE REGISTER 21
1. ALLGMEINE INFORMATIONEN 22
1.2 DAS BEDIENUNGSHANDBUCH AUFBEWAHREN 22
1.3 VERWENDETE SYMBOLE 22
1.5 ENTSORGUNG 22
2. WARNHINWEISE 22
2.1 ALLGEMEINE WARNHINWEISE 22
2.2 SPEZIFISCHE HINWEISE 23
3. BESCHREIBUNG DES PRODUKTS 24
3.2 VORGESEHENE BENUTZER 24
3.3 ERWARTETE PATIENTEN 24
3.4 HAUPTBESTANDTEILE 24
3.5 MODELLE 25
3.6 TECHNISCHE DATEN 25
3.7 BEZUGSRICHTLINIEN 25
3.8 UMWELTBEDINGUNGEN 25
4. BETRIEBSANLEITUNG 25
4.1 TRANSPORT UND LAGERUNG 25
4.2 VORBEREITUNG 25
4.3 BETRIEB 26
ABSTIEG 27
5. SCHADENSTABELLE 27
6. WARTUNG UND REINIGUNG 27
6.1 REINIGUNG 27
6.2 WARTUNG 28
6.2.1 ORDENTLICHE WARTUNG 28
6.2.2 AUSSERORDENTLICHE WARTUNG 28
28
6.3 LEBENSDAUER 28
7. ZUBEHÖR 28
8. ERSATZTEILE 28
ANHANG A - AUSBILDUNGSLOGBUCH 29
ANHANG B - WARTUNGSLOGBUCH 30
DE
EN
IT

IT
EN
13
DE
1. GENERAL INFORMATION
1.1 AIM AND CONTENTS
The aim of this manual is to supply all the information necessary so that the client, will not only attain adequate use of the appliance, he will also be capable of using the instru-
ment in the most autonomous and secure way possible. This includes information regarding technical aspects, functioning, maintenance, spare parts and safety.
1.2 CONSERVATION OF THE INSTRUCTION MANUAL
The instruction and maintenance manual must be kept together with the product, for the whole life of the device, inside the specially provided container and above all, away
from any substances or liquids which could compromise perfect legibility.
1.3 SYMBOLS USED
Symbo Meaning Symbo Meaning
The device is in compliance with the Regulation UE 2017/745 CAUTION: U.S. Federal law restricts this device to sale by or on the order of a
licensed healthcare practitioner
Medical Device Warning
Manufacturer Read user manual
Date of manufacture Serial number
UDI Unique Device Identification Product code
1.4 SERVICING REQUEST
For any information regarding the use, maintenance and installation, please contact the Spencer Customer Care Service on tel. 0039 0521 541111, fax 0039 0521 541222,
e-mail info@spencer.it or write to Spencer Italia S.r.l. – Via Provinciale, 12 - 43038 Sala Baganza (Parma) - ITALY.
In order to facilitate the assistance service, please always indicate or communicate the serial number (SN) shown on the label applied on the box or on the device.
1.5 DEMOLITION
When the devices are no more suitable for being used, if they haven’t been contaminated by any particular agents, they can be disposed of as normal solid waste, otherwise
follow the current regulations about demolition.
1.6 LABELLING
Each device has got an identifying label, positioned on the device itself and/or on the box. This label includes information about the Manufacturer, the product, CE mark, lot
number (LOT). It must never be removed or covered.
2. WARNINGS
2.1 GENERAL WARNINGS
•The product must be used by trained personnel only, having attended specific training for this device and not for similar products.
•Training routines must be registered on a special register in which the names of those trained, of the trainers, date and place are indicated. This register which will certify the
eligibility of the operators to use the Spencer device has to be kept for a period of 10 years after the disposal of the device itself. This register will be made available to the
Competent Authorities and/or Manufacturer if requested.
•Spencer Italia S.r.l. is always available for conducting training courses.
•Before carrying out any kind of operation on the appliance (training, installation, use), the operator must carefully read the enclosed instructions, paying particular attention
to the correct safety precautions and to the procedures to be followed for installation and for correct use.
•If the instructions belong to another device and not the device received, inform the Manufacturer immediately and avoid use of the device.
•In the case of any doubts as to the correct interpretation of the instructions, please contact Spencer Italia S.r.l. for any necessary clarifications.
•Do not allow untrained personnel to help when using the device as they may cause injury to the patient or themselves.
•Perform the required maintenance and to respect the life span of the device, as indicated by the Manufacturer in the User’s Manual.
•Before each use of device the perfect operating state of the device must be checked as specified in the Instruction manual. If any damage or abnormalities which could in
any way influence the correct functioning and the safety of the device, of the patient and or of the user are detected, the device must be immediately removed from service
and the Manufacturer must be contacted.
•If any failure or incorrect functioning of the device is detected, it must be immediately substituted with a similar item so that the rescue procedures are guaranteed without
any interruption.
•Use of the device in anyway other than described in this manual is forbidden.
•Do not alter or modify in any way the appliance; any such interference could cause malfunctions and injury to the patient and/or rescuer.
•The appliance must not in any way be tampered with (modification, adjustment, addition, replacement). In such cases all responsibility will be denied for any malfunctions or
injuries caused by the appliance itself; moreover CE certification and product warranty will be considered void.
•Those who modify or have modified, prepare or have prepared medical appliances in such a way that they no longer serve the purpose for which they were intended, or no
longer supply the intended service, must satisfy the valid conditions for the introduction onto the market.
•Handle with care.
•Ensure that all the necessary precautions are taken in order to avoid the hazards that can arise as the result of contact with blood or body fluids.
•Register and store with these instructions: lot number, place and date of purchase, first date of use, date of checks, name of users, any comments.
•When the device is being used, the assistance of qualified staff must be guaranteed.
•Never leave an unassisted patient. The presence of at least one operator is essential at all times when the medical device is in use.
•Do not store the device underneath any heavy objects which could cause structural damage.
•Store in a cool, dry, dark place and do not expose to direct sun.
•Store and transport device in its original packaging.
•The device not be exposed to or come into contact with any source of combustion or inflammable agents.
•Position and adjust the device taking care not to cause any obstruction to rescuers and or any other rescue equipment.
•Attention: laboratory testing, post production tests, instruction manuals cannot always consider every possible scenario for use. This means that in some cases the perfor-
mance of the product could be notable different from results to date obtained. Instructions are continually being updated and are under tight surveillance of fully qualified
staffs with adequate technical formation.
•Avoid contact with the skin by placing a surgical cloth between the patient and the device.
With reference to the Regulation UE 2017/745, we remind both public and private operators that they are obliged to report any accident that involves any medical device
to the Ministry of Health and to the Manufacture as specified and within time given by the European regulations.
In addition, both public and private operators are obliged to inform the Manufacturer of any measures that should be adopted to make the steps necessary to guarantee
the safety and the health of the patients and the users o any medical device.

14
IT
EN
DEDE
2.2 SPECIFIC WARNINGS
•Establish a maintenance program and periodic testing, identifying an reference employee. The person to whom the ordinary maintenance of the device is entrusted must
ensure the basic requirements foreseen by the Manufacturer in the user’s manual.
• Training routines must be registered on a special register in which the names of those trained, of the trainers, date and place are indicated. This register which will certify the
eligibility of the operators to use the Spencer device has to be kept for a period of 10 years after the disposal of the device itself. This register will be made available to the
Competent Authorities and/or Manufacturer if requested.
•If any failure or incorrect functioning of the device is detected, it must be immediately substituted with a similar item so that the rescue procedures are guaranteed without
any interruption.
•Before each use of device the perfect operating state of the device must be checked as specified in the Instruction manual. If any damage or abnormalities which could in
any way influence the correct functioning and the safety of the device, of the patient and or of the user are detected, the device must be immediately removed from service
and the Manufacturer must be contacted.
•When the device is being used, the assistance of qualified staff must be guaranteed.
•Never leave an unassisted patient. The presence of at least one operator is essential at all times when the medical device is in use.
•The device not be exposed to or come into contact with any source of combustion or inflammable agents.
•Store in a cool, dry, dark place and do not expose to direct sun.
•Do not store the device underneath any heavy objects which could cause structural damage.
•Store and transport device in its original packaging.
•Position and adjust the device taking care not to cause any obstruction to rescuers and or any other rescue equipment.
•For blocking and transporting the patient, follow the procedures approved by the Emergency Medical Service.
•Always respect the maximum load capacity, indicated in this user’s manual.
•Do not operate if the weight is not correctly distributed.
•
•When using the device for evacuation over stairways or simple transport, at least two operators with suitable physical conditions are needed.
2.3 CONTRAINDICATIONS AND SIDE EFFECTS
The use of this device, if used as described in this manual, does not present any contraindications or collateral effects.
2.4 PHYSICAL REQUIREMENTS OF THE OPERATORS
Operators using the device must possess the following minimum requirements:
•physical capacity for using the device.
•be able to grab the device firmly with both hands
•have robust back, arms and legs for lifting, pushing and pulling the evacuation chair
•have a good muscular coordination
Every operator has to be trained in safe and efficient patient transport techniques.
Loading techniques, in case of extremely heavy patients, uneven terrains or unusual situations, may require more than the usual two operators.
Before deciding the roles of the operators during the use of the device, their capabilities must be evaluated.

IT
EN
15
DE
3. PRODUCT DESCRIPTION
3.1 INTENDED USE
The stair chairs are devices to be used to move and bring a patient into a sitting position to the ambulance, but not to be used for transporting the patient inside the ambulance.
They are equipped with slides that assist the descent of the stairs in order to reduce the fatigue required by the operator during the transfer of patient and chair. They must
not be used for parking.
3.2 INTENDED USERS
The intended users are people trained in evacuation procedures in emergency conditions.
Among the possible users are also contemplated the fitters of the environments in which the chair is expected to be positioned when not in use.
3.3 PATIENTS
The patient must have an anatomy and a condition such as to allow the transport in a sitting position with thighs and back adequately supported and belts well fastened.
The rescuer is the person responsible for assessing the suitability of the device for use on the specific patient.
There are no known contraindications or side effects resulting from the use of the device, as long as it is used in accordance with the user manual.
3.4 MAIN COMPONENTS
N° COMPONENT MATERIAL SKID-E PRO
SKID-E
PRO SKID-E
MAX
PRO SKID-E
AIR
SKID-E
READY SKID-OK SKID-OK
MAX
1 Front footrest Aluminium
2 Rear left handle group Aluminium
3 Rear right handle group Aluminium
4 Front telescopic left handle group Aluminium
5 Front telescopic right handle group Aluminium
6 Blocking system rear handles (n° 2) Aluminium
7 Seat sheet PVC
8 Backrest sheet PVC
Extendible handle Aluminium
10 Rear wheels Ø 200 mm (n° 2) Rubber coated
polyurethane
11 Front wheels Ø 100 mm pivoting with
brake (n° 2) Polypropylene
12 Slide with belt (n° 2) Covered rubber
13 Rear wheels Ø 200 x 32 mm (n° 2) Polypropylene
14 Front wheels Ø 150 x 32 mm (n° 2) Rubber coated
polyurethane
fig. A fig. B

16
IT
EN
DEDE
3.5 MODELS
Evacuation chair with silver frame and black sheet
Evacuation/transport chair with yellow frame and black sheet
Evacuation/transport chair with silver frame and black sheet
Evacuation chair with fixed backrest, yellow frame and black sheet
Evacuation chair
Evacuation chair with yellow frame and black sheet
Load capacity 250 kg
Load capacity 250 kg
3.6 TECHNICAL DATA
CHARACTERISTICS SKID-E PRO SKID-E PRO SKID-E MAX PRO SKID-E AIR SKID-E READY SKID-OK SKID-OK MAX
Width (mm) 530 550 550 410 530 520 520
Length (mm) 1110 1110 1110
Length with opened handles (mm) – 1450 1450 1450 – – –
Height with opened backrest (mm) 1600 1600 1600 1600 1600 1540 1540
Height with closed backrest (mm) 1070 1070 1070 1070 – 1040 1040
Thickness closed (mm) 330 330 330 330 330 175 175
Weight (kg) 12,7 14,2 14,2 13,8 13,5 10 10
Maximum load capacity (kg) 150 150 250 150 150 150 250
3.7 REFERENCE STANDARDS
REFERENCE TITLE OF DOCUMENT
Regulation 2017/745/UE European Regulation of the european parliament and of the council of 5 April 2017 on medical devices
3.8 ENVIRONMENTAL CONDITIONS
Functioning temperature: from -15 to +50 °C
Storage temperature: from -20 to +60 °C
4. OPERATING INSTRUCTIONS
4.1 TRANSPORT AND STORAGE
Before transporting the appliance, make sure that it is correctly packaged ensuring also that there are no risks of shocks, bumps or falls during the transport itself.
-
rantee. Repairs or replacement of the damaged parts are the responsibility of the client. The device must be stored in a dry, cool area away from direct sunlight. It must not be
placed in contact with any substances or chemical agents which could cause damage and reduce safety characteristics.
During storage take care not to put heavy materials onto the device. In no way and under no circumstances should the device be considered as a work top.
4.2 PREPARATION
On receipt of the product:
•Remove the packaging and display the material so that all components are visible.
•Check that all the components/pieces on the accompanying list are present.
The appliance must be checked before every use so as to reveal any working abnormalities and/or damage caused by transport and/or storage.
Therefore, before using the device, check:
•General functionality of the device
•Fixation of nuts and bolts
•State of use of the wheels and braking system
•State of use of the caterpillar belts and slide
•State of use of restraints, seat and headrest
•Functionality springs
If the device respects these conditions, it may be considered ready for use.

IT
EN
17
DE
4.3 FUNCTIONING
4.3.1 OPENING THE DEVICE
1. Put the device in vertical position (fig. C) and pull
out the extendible rear handle until it is blocked au-
tomatically using the purpose made locking hooks
model).
2. Loosen the restraints, to free the “chair”.
3. Grab with one hand the seat and the extendible
handle with the other, push both parts away from
each other, until the chair is completely opened. The
evacuation chair is now ready to be placed safely on
the floor
4. Unblock the brakes of the two front Ø 100 mm whe-
els. The device is now ready for use.
4.3.2 CLOSING THE DEVICE
If the device is not occupied by the patient:
1 - Using both breaks, block both front Ø100 mm wheels turned in inside direction.
2 - Only PRO SKID-E and PRO SKID-E MAX models: Disengage the rear handles (if positioned as in fig. E - F) by inserting the hooks in the blocking system (fig. G).
3 - Take position at the rear side of the chair, push both levers in order to unblock the extending handle and push it downwards along the special nylon guides.
4 - Grab the “seat” and the backrest and push them towards one another until the closed position is reached.
5 - Attach the belt around the body which will block the seat in position.
fig. C
fig. E fig. F fig. G
fig. D

18
IT
EN
DEDE
4.3.3 Patient transport on stairways - Downstairs
1 - Apply the procedure described in paragraph 4.3.1.
2 - Grab the main handle firmly with both hands and let the patient sit on the chair. Make sure during this opera-
tion that both front Ø 100 mm wheels touch the ground.
3 - Secure the patient to the device using the special safety belts for the head and the body.
4 - Move close to the stairs and position on the side with the handrail. The first operator always keeps his hands
on the handle firmly and follows the downstairs movement of the device, maintaining continual speed control
and grip for a safe downstairs movement.
The second operator should not be in front of the device (in front of the patient), but on the side of the device
and to a certain distance (a few steps down), he must drive down operations in an optimal manner and must
be prepared to intervene if necessary, without ever compromising his safety.
5 - Maintain a constant pressure in downward direction. This will improve the stability of the device (fig. H).
6 - When the descent has terminated, position the wheels correctly on the surface in order to guarantee safe
and easy horizontal maneuvering.
4.3.4 Patient transport on stairways - Upstairs (PRO SKID-E and PRO SKID-E MAX MODELS ONLY)
1 - Apply the procedure described in paragraph 4.3.1.
2 - Secure the patient to the device using the special safety belts for the head and the body.
3 - The first operator behind the device, has to block the rear handles (fig. G), rotating the hook downwards and
fixing it to the hinge between slide and frame (fig. E - F).
4 - The second operator, in front of the chair, has to grab the front telescopic handles at the front and move them
by pushing the button.
5 - Using adequate lifting techniques, the operators must simultaneously lift the chair and start transport (fig. I).
For this type of transport the presence of a third operator is recommended in order to guide the other
two.
5. TROUBLESHOOTING
PROBLEM CAUSE REMEDY
The device does not unblock from open or closed
position during the opening or closing procedure.
The functional geometry has been compromised or
damaged.
Try complete lubrication and check if the problem has
been solved; if not, take immediately the device out of
service and contact the Spencer customer care service
Difficulties extracting and inserting the telescopic
models).
Presence of foreign bodies in the slide or breakage in
aluminium part.
Clean accurately; if this does not solve the problem, do
not use the device for upstairs transport and contact
the Spencer customer care service.
The headrest extendible handle does not block in
open position. Breakage of springs of the blocking system. Take the device immediately out of service and contact
the Spencer customer care service.
Difficulties in controlling the device during
downstairs transport. Damage of the sliding belt system. Take the device immediately out of service and contact
the Spencer customer care service.
Structural damage. Improper use or untrained personnel. Take the device immediately out of service and contact
the Spencer customer care service.
6. MAINTENANCE AND CLEANING
6.1 CLEANING
Failure to carry out cleaning operations may involve the risk of cross infection due to the presence of secretions and/or residuals.
The operator must always wear adequate personal protection such as gloves and mask etc. during all checking and cleaning procedures.
The metal parts exposed to external influences are treated superficially and/or painted in order to obtain better resistance. Clean the exposed parts with delicate soap and
a sponge and dry with a soft cloth. In order to obtain a shiny finish of the frame parts, use shiny creams and waxes for vehicles. We advice the use of the polishing detergent
Do not use high-pressure water. It may penetrate joints and eliminate lubricants, increasing the risk of corrosion of components.
Rinse carefully with warm water to remove all traces of detergents. Failure to do so could compromise the product and its life span. The device must be left to dry completely
before storage. To dry the product after washing, or if used in a humid atmosphere, do not use direct sources of heat or flame.
6.2 MAINTENANCE
Establish a maintenance program and periodic testing, identifying an reference employee. The person who carries out the maintenance of the appliance has to guarantee the
basic requirements indicated by the Manufacturer in the following paragraphs.
All maintenance activities, both precautionary and special, must be registered on documents including technical reports about operations. This register has to be kept for a
period of at least 10 years after the disposal of the device itself. This register will be made available to the Competent Authorities and/or Manufacturer if requested.
With reference to the Regulation UE 2017/745, we remind both public and private operators that they are obliged to report any accident that involves any medical device
to the Ministry of Health and to the Manufacture as specified and within time given by the European regulations. In addition, both public and private operators are
fig. H
fig. I

IT
EN
DE
obliged to inform the Manufacturer of any measures that should be adopted to make the steps necessary to guarantee the safety and the health of the patients and
the users o any medical device.
6.2.1 PRECAUTIONARY MAINTENANCE
The person who carries out the precautionary maintenance of the appliance (user in person, Manufacturer/supplier or a third party) has to guarantee the following basic
requirements:
•Technical knowledge of the appliance and of the periodic maintenance procedures as described in these instructions.
•Specific qualifications and training in the maintenance operations of the appliance in question.
•The use of components/replacement parts/accessories that are either original or approved by the supplier, in such a way that each operation causes no alteration or modi-
fication to the appliance.
•Possession of the checklist of operations carried out on the appliance.
•Guarantee complete adherence to the instructions of the Regulation UE 2017/745 which includes also the obligation towards the Manufacturer to maintain post sales records
and traceability of the appliance if requested.
During all checking, maintenance and cleaning procedures, the operator must wear adequate personal protection such as gloves, mask, glasses etc.
Check ups to be performed before every use:
•Functionality of the device
•Fixation of nuts and bolts
•State of use (moving parts, wheels, restraints, seat, headrest, sliding system, belts)
•Correct lubrication of moving parts
The inspection frequency is determined by factors such as legal requirements, the type of use, frequency of use, environmental conditions during use and storage.
Please note that you must do the cleaning as described in paragraph 5.1 and verify functionality before and after each use. Spencer Italia S.r.l. declines any responsibility for
the unproper functioning or damages caused to the patient or user by the use of devices not subject to routine maintenance, warranty and will void the compliance to the
Medical Device Regulation UE 2017/745.
The person responsible for every day maintenance can only substitute the spare parts indicated on paragraph 6.2 “Spare Parts”. All other substitutions or repairs can
be carried out only by the manufacturer or by a centre authorised by the manufacturer.
6.2.2 SPECIAL SERVICING
Only the Manufacturer or centres with written authorisation are authorised to complete any special servicing operations.
For any operations that are not carried out directly by the Manufacturer but by an authorised centre, we have to underline that a report regarding all operations carried out
must be requested. This will permit both Spencer Italia S.r.l. and the end user to keep a log book regarding the operations carried out on the device.
6.2.3 PERIODIC MAINTENANCE
The device must be subjected to annual revisions to verify the proper operation and compliance with the safety requirements guaranteed by the Manufacturer when the
device is placed on the market.
The revisions must be made by the Manufacturer, who uses specialized internal and external technicians and is authorized by the Manufacturer himself. In the absence of such
annual revisions, the device must be SECRETED UNTIL REPAIRING, otherwise it must be DISPOSED OF AND IT MUST BE GIVEN COMMUNICATION TO THE MANUFACTURER.
For any operations that are not carried out directly by the Manufacturer but by an authorised centre, we have to underline that a report regarding all operations carried out
must be requested. This will permit both Spencer Italia S.r.l. and the end user to keep a log book regarding the operations carried out on the device.
6.3 LIFE SPAN
The device, if used as described in this user manual, has an average life span of 5 years from the date of purchase, which can be extended following annual revisions.
The life span can be extended, based on manufacturer’s or on authorized service center evaluation, if the safety requirements of the device are still guaranteed.
In the absence of such extensions, the device must be DISPOSED AND IT MUST BE COMMUNICATED TO THE MANUFACTURER.
Belts, fabric seat and backrest shall be replaced every two years.
Spencer Italia S.r.l. disclaims any responsibility for incorrect operation or for any damage caused by the use of devices not revised by the Manufacturer or authorized service
center, or that have exceeded the maximum permissible life span.
7. ACCESSORIES
Wall bracket
Strap for ankles
8. SPARE PARTS
Backrest sheet ST30428B Lower wheel carrier in black nylon
Seat sheet Higher wheel carrier in black nylon
ST00427A Pair of black belts with Derlin buckle Black PVC handle
ST21400A 100 mm Ø wheel with brake
Warning
The information contained in this document could be modified without any warning and is not to be intended as a commitment on behalf of Spencer Italia S.r.l. Spencer
products are exported to many countries and the same identical regulations are not always valid. For this reason there could be differences between the description here
described and the product actually delivered. Spencer continually strives to reach the perfection of all items sold. We therefore hope you will understand if we reserve the
right, at any time, to modify the shape, equipment, lay-out or technical aspects that are herein described.
© Copyright Spencer Italia S.r.l.
All rights reserved. No part of this document can be photocopied, reproduced or translated into another language without the written approval of Spencer Italia S.r.l.

20
IT
EN
DEDE
ATTACHMENT A – TRAINING REGISTER
The product must be used by trained personnel only, having attended specific training for this device and not for similar products.

IT
EN
21
DE
ATTACHMENT B – MAINTENANCE REGISTER
Perform the required maintenance and to respect the life span of the device, as indicated by the Manufacturer in the User’s Manual.
Code and description of the device
Purchase date
Lot (LOT)
Bought by

22
IT
EN
DEDE
1. ALLGMEINE INFORMATIONEN
1.1 ZWECK UND INHALT
Sicherheit.
1.2 DAS BEDIENUNGSHANDBUCH AUFBEWAHREN
1.3 VERWENDETE SYMBOLE
Symbol Bedeutung Symbol Bedeutung
entspricht Allgemeine Warnhinweise oder Spezifikationen
Im Handbuch nachsehen
Hersteller Seriennummer
Herstellungsdatum Produktcode
UDI
1.4 KUNDENDIENSTANFRAGE
Sie sich bitte an den Kundendienst von Spencer, Tel. 0039
0521 541111, Fax 0039 0521 541222, E-Mail info@spencer.it oder schreiben Sie an Spencer Italia S.r.l. Via Provinciale Nr. 12 – 43038 Sala Baganza (Parma) - ITALIEN.
geben Sie immer die Seriennummer (SN) auf dem Etikett
diese mit.
1.5 ENTSORGUNG
1.6 KENNZEICHNUNG
Dieses Schild darf niemals entfernt oder abgedeckt werden.
2. WARNHINWEISE
2.1 ALLGEMEINE WARNHINWEISE
•
•Die Schulungen werden in ein spezielles Register eingetragen, in dem die Namen der geschulten Personen, der Ausbilder, das Datum und der Ort angegeben werden. Diese
•
•-
ders auf die entsprechenden Sicherheitsvorkehrungen sowie die Installations- und Verwendungsmethoden achten.
•
dem Hersteller in Verbindung setzen.
•
•
•
•
und der Hersteller kontaktiert werden.
•
•
•-
•
•
•Vorsichtig handhaben.
•
•
•
•
•
•
•
•
•
•-
gen, daher können Ergebnisse unter realen Gebrauchsbedingungen des Produkts im Einsatzfeld mitunter deutlich von ihnen abweichen. Die beste Anleitung ist der stete
Gebrauch unter Aufsicht von erfahrenem und ausgebildetem Personal.
•

IT
EN
23
DE
2.2 SPEZIFISCHE HINWEISE
•ordentliche Wartung
anvertraut ist, muss die vom Hersteller vorgesehenen Grundanforderungen im Rahmen dieser Gebrauchsanweisungen sicherstellen.
•-
•
•
und der Hersteller kontaktiert werden.
•
•
•
•
•
•
•
•
•
•Nicht handhaben, wenn das Gewicht nicht gut verteilt ist.
•
•-
tienten zu verwenden.
2.3 GEGENANZEIGEN UND NEBENWIRKUNGEN
2.4 KÖRPERLICHE VORAUSSETZUNGEN DER EINSATZKRÄFTE
bestimmt sind.
•
•
•
•
-
hen Situationen können die Verladetechniken den Einsatz von mehr als zwei Bedienern erfordern.
Die Fähigkeiten jedes Anwenders müssen vor Festlegung der Aufgabenverteilung der Einsatzkräfte beim Einsatz des Geräts geprüft werden.

24
IT
EN
DEDE
3. BESCHREIBUNG DES PRODUKTS
3.1 VERWENDUNGSZWECK
-
3.2 VORGESEHENE BENUTZER
3.3 ERWARTETE PATIENTEN
-
3.4 HAUPTBESTANDTEILE
N° BESTANDTEIL MATERIAL SKID-E PRO
SKID-E
PRO SKID-E
MAX
PRO SKID-E
AIR
SKID-E
READY SKID-OK SKID-OK
MAX
1 Aluminium
2 Griff-Gruppe hinten links Aluminium
3 Griff-Gruppe hinten rechts Aluminium
4 Teleskopgriff-Gruppe vorne links Aluminium
5 Teleskopgriff-Gruppe vorne rechts Aluminium
6 Halterung Teleskopgriffe hinten (Nr. 2) Aluminium
7 Sitzbezug PVC
8 PVC
Ausziehbarer Griff Aluminium
10 Hinterrad Ø 200 mm (Nr. 2) gummiertes
Polyurethan
11 Schwenkbares Vorderrad Ø 100 mm
mit Bremse (Nr. 2) Polypropylen
12 Riemenschlitten (Nr. 2) Stoff Gummi
13 Hinterad Ø 200 x 32 mm (Nr. 2) Polypropylen
14 Hinterad Ø 150 x 32 mm (Nr. 2) gummiertes
Polyurethan
Abb. A Abb. B

IT
EN
25
DE
3.5 MODELLE
Evakuierungsstuhl mit silbernem Rahmen und schwarzer Bezug
Evakuierungs-/Transportstuhl mit gelbem Rahmen und schwarzer Bezug
Evakuierungs-/Transportstuhl mit silbernem Rahmen und schwarzer Bezug
Evakuierungsstuhl
Evakuierungsstuhl mit gelbem Rahmen und schwarzer Bezug
3.6 TECHNISCHE DATEN
MERKMALE SKID-E PRO SKID-E PRO SKID-E MAX PRO SKID-E AIR SKID-E READY SKID-OK SKID-OK MAX
Breite (mm) 530 550 550 410 530 520 520
1110 1110 1110
– 1450 1450 1450 – – –
Höhe mit ausgezogenem Griff (mm) 1600 1600 1600 1600 1600 1540 1540
Höhe mit geschlossenem Griff (mm) 1070 1070 1070 1070 – 1040 1040
Dicke geklappt (mm) 330 330 330 330 330 175 175
Gewicht (kg) 12,7 14,2 14,2 13,8 13,5 10 10
Maximale Traglast (kg) 150 150 250 150 150 150 250
3.7 BEZUGSRICHTLINIEN
BEZUG TITEL DES DOKUMENTS
Verordnung EU 2017/745
3.8 UMWELTBEDINGUNGEN
Betriebstemperatur: -15 bis +50 °C
Lagertemperatur: -20 bis +60 °C
4. BETRIEBSANLEITUNG
4.1 TRANSPORT UND LAGERUNG
4.2 VORBEREITUNG
Bei Erhalt des Produkts:
•Entfernen Sie die Verpackung und ordnen Sie das Material gut sichtbar an.
•
•
•Anzug der Schrauben und Bolzen
•
•
•Abnutzungszustand von Riemen und Blechen
•Schnappen der Federn

26
IT
EN
DEDE
4.3 BETRIEB
4.3.1 ÖFFNEN DES GERÄTS
ziehen Sie ihn heraus, bis er mit Hilfe der Verriegelun-
2. Lösen Sie die Rumpfsicherheitsgurte, um den “Sitz”
zu entriegeln.
den “Sitz” mit der anderen (Abb. D) und öffnen Sie
bis sich die automatische Verriegelung in der offenen
Position befindet. Der Tragesessel kann nun sicher auf
dem Boden abgestellt werden.
4. Lösen Sie die Bremse der beiden Ø 100 mm
einsatzbereit.
4.3.2 SCHLIESSEN DES GERÄTS
1 - Verriegeln Sie die beiden Ø 100 mm-Lenkrollen mit ihren Bremsen, nachdem Sie sie nach innen gedreht haben.
2 - Nur für die Modelle PRO SKID-E und PRO SKID-E MAX
-
schlossenen Position befinden.
5 - Befestigen Sie den Rumpfgurt, um den “Sitz” zu fixieren.
Abb. C
Abb. E Abb. F Abb. G
Abb. D

IT
EN
27
DE
4.3.3 Transport von Patienten auf Treppen - Abstieg
1 - Wenden Sie das in Abschnitt 4.3.1 beschriebene Verfahren an.
Vorgang darauf, dass beide Ø 100 mm Lenkrollen auf dem Boden stehen.
-
Der zweite Bediener darf niemals vor dem Gerät (vor dem Patienten) stehen, sondern
muss seitlich des Geräts und in einem gewissen Abstand (einige Schritte nach unten) die Abseilvorgänge opti-
mal leiten und bereit sein, bei Bedarf einzugreifen, ohne jemals seine eigene Sicherheit zu gefährden.
6 - Wenn Sie das Ende der Treppe erreicht haben, bringen Sie den Stuhl wieder in eine aufrechte Position, so dass
4.3.4 Transport von Patienten auf Treppen - Aufstieg (NUR PRO SKID-E und PRO SKID-E MAX MODELL)
1 - Wenden Sie das in Abschnitt 4.3.1 beschriebene Verfahren an.
dem Stuhl.
Verriegelung gelöst hat (Abb. G), indem er den Haken nach unten dreht und ihn dann auf dem Bolzen zwischen
beginnen (Abb. I).
Für diese Art von Transport wird die Anwesenheit eines dritten Bedieners empfohlen, um die Operatio-
nen der ersten beiden optimal zu steuern.
5. SCHADENSTABELLE
PROBLEM URSACHE ABHILFE
beschlagnahmt
Wenn das Problem weiterhin besteht, nehmen Sie
Schwierigkeiten beim Herausziehen und
Vorhandensein von Ablagerungen im Schiebesitz oder
Versagen des Aluminiumprofils
nicht zum Treppensteigen und wenden Sie sich an den
in der ausgezogenen Position eingerastet
beim Heruntersteigen von Treppen
der Schlitten
Personal
6. WARTUNG UND REINIGUNG
6.1 REINIGUNG
Während aller Kontroll- und Hygienemaßnahmen muss der Bediener geeignete persönliche Schutzausrüstung wie Handschuhe, Schutzbrille usw. tragen.
ausgesetzten Teile mit lauwarmem Wasser und neutraler Seife waschen; niemals Lösungsmittel oder Fleckentferner verwenden. Zur Desinfektion Produkte verwenden, die
Um einen hohen Glanz auf Rahmenteile zu erzielen, empfehlen wir Cremes oder Wachse, die zum Polieren von Autokarosserien verwendet werden.
seine Lebensdauer verringern könnte.
Abb. H
Abb. I

28
IT
EN
DEDE
6.2 WARTUNG
Bediener öffentlicher oder privater Gesundheitsdienste müssen den Hersteller über alle anderen Störungen
informieren, die es ermöglichen, Maßnahmen zum Schutz und zur Sicherstellung der Gesundheit von Patienten und Anwendern zu ergreifen.
6.2.1 ORDENTLICHE WARTUNG
•
•
•
•
•
Während aller Kontroll-, Wartungs- und Hygienemaßnahmen muss der Bediener geeignete persönliche Schutzausrüstung wie Handschuhe, Schutzbrille usw. tragen.
•
•Anzug Schrauben und Bolzen
•
•Schmierung der bewegten Teile
Die Häufigkeit der Kontrollen richtet sich nach Faktoren, wie die gesetzlichen Bestimmungen, die Art des Gebrauchs, die Häufigkeit des Gebrauchs, die Umwelt-
bedingungen während der Verwendung und die Lagerung.
Die mit der ordentlichen Wartung beauftragte Person darf nur die im Abschnitt “6.2 Ersatzteile” angegebenen Ersatzteile ersetzen. Für andere Austausch-/Reparatu-
rarbeiten wenden Sie sich bitte an den Hersteller oder an eine vom Hersteller autorisierte Stelle.
6.2.2 AUSSERORDENTLICHE WARTUNG
Bei Eingriffen, die nicht vom Hersteller, sondern von einem autorisierten Zentrum durchgeführt werden, erinnern wir Sie daran, dass Sie einen Bericht über die durch-
geführte Tätigkeit anfordern müssen. Auf diese Weise können sowohl Spencer Italia S.r.l. als auch der Nutzer alle durchgeführten Eingriffe im Laufe der Zeit nachvollziehen.
6.2.3 REGELMÄSSIGE ÜBERHOLUNG
-
6.3 LEBENSDAUER
7. ZUBEHÖR
Wandhalterung
8. ERSATZTEILE
ST30428B Unterer Radhalter aus schwarzem Nylon
Sitzbezug Oberer Radhalter aus schwarzem Nylon
ST00427A
ST21400A Rad Ø 100 mm mit Bremse

IT
EN
DE
ANHANG A - AUSBILDUNGSLOGBUCH

30
IT
EN
DEDE
ANHANG B - WARTUNGSLOGBUCH
Gerätecode und Beschreibung
Kaufdatum
Charge (LOS) oder Seriennummer (SN)
Gekauft von
Hinweis
hier vereinbarten Angaben vorzunehmen.
© Copyright Spencer Italia S.r.l.
This manual suits for next models
5
Table of contents
Languages:
Other Spencer Safety Equipment manuals
Popular Safety Equipment manuals by other brands
Lanex
Lanex PB-20 instruction manual

SKYLOTEC
SKYLOTEC ANCHOR ROPES Instructions for use

Besto
Besto Buoyancy Aid 50N Instructions for use

TEUFELBERGER
TEUFELBERGER NODUS Manufacturer's information and instructions for use

Troy Lee Designs
Troy Lee Designs Tbone Product owners manual

Innova
Innova Xtirpa Instruction and safety manual

bolle SAFETY
bolle SAFETY B810 quick start guide
SHENZHEN FANHAI SANJIANG ELECTRONICS
SHENZHEN FANHAI SANJIANG ELECTRONICS A9060T instruction manual

Hiltron security
Hiltron security POWER8E Installation and use manual

Salewa
Salewa MTN SPIKE user manual
Hatco
Hatco B-950P installation guide

Sitec
Sitec TX MATIC operating manual