SphygmoCor XCEL User manual

Service Manual
SphygmoCor XCEL System

Contents
Cautions, Warnings and Contraindications ..............................................................................4
Troubleshooting SphygmoCor XCEL ........................................................................................5
SphygmoCor XCEL Device.....................................................................................................5
The Tonometer fails to respond..............................................................................................5
SphygmoCor XCEL Software .................................................................................................6
Error Codes.............................................................................................................................6
Database Manager ...............................................................................................................13
Backup a Database .......................................................................................................13
Restore a Database.......................................................................................................14
Basic System Care ...............................................................................................................16
Stability ..........................................................................................................................16
Pressure or impact damage...........................................................................................16
Temperature ..................................................................................................................16
Magnetic fields...............................................................................................................16
Liquids............................................................................................................................16
Weight............................................................................................................................16
Movement......................................................................................................................16
Shutting down................................................................................................................17
Equipment Calibration ...................................................................................................17
Cleaning Instructions ............................................................................................................17
Notebook Batteries ........................................................................................................17
Tonometer Care.............................................................................................................17
Disinfection Instructions for Tonometer and Cuffs.........................................................17
PWV Thigh Cuff Care ....................................................................................................18
PWA Brachial Cuff Care .......................................................................................................18
Cleaning instructions .....................................................................................................18
Calibrating SphygmoCor XCEL................................................................................................19
SphygmoCor XCEL Calibration Kit Operating Instructions...................................................19
Calibration Setup ..................................................................................................................21
Installation of the SphygmoCor XCEL Calibration Software.................................................22
SphygmoCor XCEL Calibration Steps..................................................................................24
Calibration Troubleshooting..................................................................................................44
Finding Com Port...........................................................................................................44
Restarting the Calibration Procedure ............................................................................45
Spare Parts List..........................................................................................................................46
Technical Specifications...........................................................................................................48
Physical and Environmental Specifications ..........................................................................48
Maximum Intended Design Life ............................................................................................48
Minimum Computer Requirements.......................................................................................49
Classification of SphygmoCor System..................................................................................49
Standards..............................................................................................................................50

Copyright
SphygmoCor®XCEL Service Manual
Copyright © 2013 AtCor Medical Pty. Ltd., Sydney Australia. All rights reserved. Under the copyright
laws, this manual cannot be reproduced in any form without prior written permission of AtCor Medical
Pty. Ltd.
DCN: 101397
Manual Revision: 4.0
Head Office:
AtCor Medical Pty Ltd
West Ryde Corporate Centre
Suite 11, 1059-1063 Victoria Rd.
West Ryde NSW 2114
Sydney, Australia
Telephone: + (61) 2 9874 8761
Facsimile: +(61) 2 9874 9022
Email: inquiry@atcormedical.com
Web: www.atcormedical.com
USA Office and US FDA Agent:
AtCor Medical Inc
One Pierce Place,
Suite 255W-,
Itasca, IL, 60143,
USA
Telephone: + (1) 630 228 8871
Facsimile: + (1) 630 228 8872
Email: atcorusa@atcormedical.com
European Authorized Representative:
Advena Ltd
Thorne Widgery House
33 Bridge Street
Hereford HR4 9DQ
United Kingdom
Telephone: + (44) 845 094 3307
Facsimile: + (44) (0) 156 862 0078
Disclaimer
This manual has been validated and reviewed for accuracy. The instructions and descriptions it contains
are accurate for the AtCor Medical product models at the time of this manual’s production. However,
succeeding models and manuals are subject to change without notice. AtCor Medical assumes no
liability for damages incurred directly or indirectly from errors, omissions or discrepancies between the
product and the manual.
This Manual is produced on the assumption that the operator is an experienced user of the Windows
XP/ Windows 7/ Windows 8 operating Systems.
If the operator is not familiar with Windows operations, please refer to the Online Help of Windows or
the Windows User Manual.
Trademarks
“SphygmoCor®” is a registered trademark of AtCor Medical Pty Ltd.
IBM, Microsoft, and Windows are the registered trademarks of their respective holders.
Caution
Federal (USA) law restricts this device to sale by or on the order of a physician
Cautions, Warnings and Contraindications
Please refer to the SphygmoCor XCEL Operator’s Manual (DCN 101335) for the list of cautions, warnings
and contraindications.

Page 5
Troubleshooting SphygmoCor XCEL
Caution: If any error messages indicate "database corruption" or "database access error" contact AtCor
Medical Technical Support immediately.
The SphygmoCor XCEL Software conducts a series of internal tests with prompts to guide the user
should an error occur. In most instances, errors can be resolved following these prompts. However, if
the error does not resolve or the solution is not apparent, please contact AtCor Medical Technical
Support for assistance.
SphygmoCor XCEL Device
Condition 1: The SphygmoCor XCEL device cannot be detected.
This error may appear at two places in the software:
When the SphygmoCor XCEL software is started.
Upon entry into the Capture Screen.
Check the following items:
The SphygmoCor XCEL device is connected to your computer.
The SphygmoCor XCEL device is plugged into an AC outlet and the power switch is toggled to the
“on” position.
The Power light is illuminated (if it is not illuminated, refer to Condition 2 “The POWER light is off”
below)
The correct communications port is selected in the configuration settings.
From the Setup Screen, select System –Find Module.
If using a USB Adaptor, ensure that the USB drivers have been installed on the computer (refer to
Step 4 in Installation on the User Manual).
If none of the above actions resolves the issue, please contact AtCor Medical Technical Support.
Condition 2: The POWER light is off.
Check the following:
The SphygmoCor XCEL device power cable is connected to an AC outlet and the toggle switch is in
the “ON” position.
If the above action does not resolve the issue, please contact AtCor Medical Technical Support.
Note: If the Status Light is either flashing yellow, flashing red, or is off, then return the unit to the
manufacturer.
The Tonometer fails to respond.
Ensure that the Tonometer is connected to the appropriate port at the rear of the SphygmoCor XCEL
device.
Note: Use caution when connecting the Tonometer as pins inside the connector can be easily bent or
broken.
Page 6
SphygmoCor XCEL Software
The user will be notified of software issues via pop-up windows with instructions to resolve the issue. If
the screen freezes or you require further explanation of any pop-up window, please contact AtCor
Medical Technical Support for assistance.
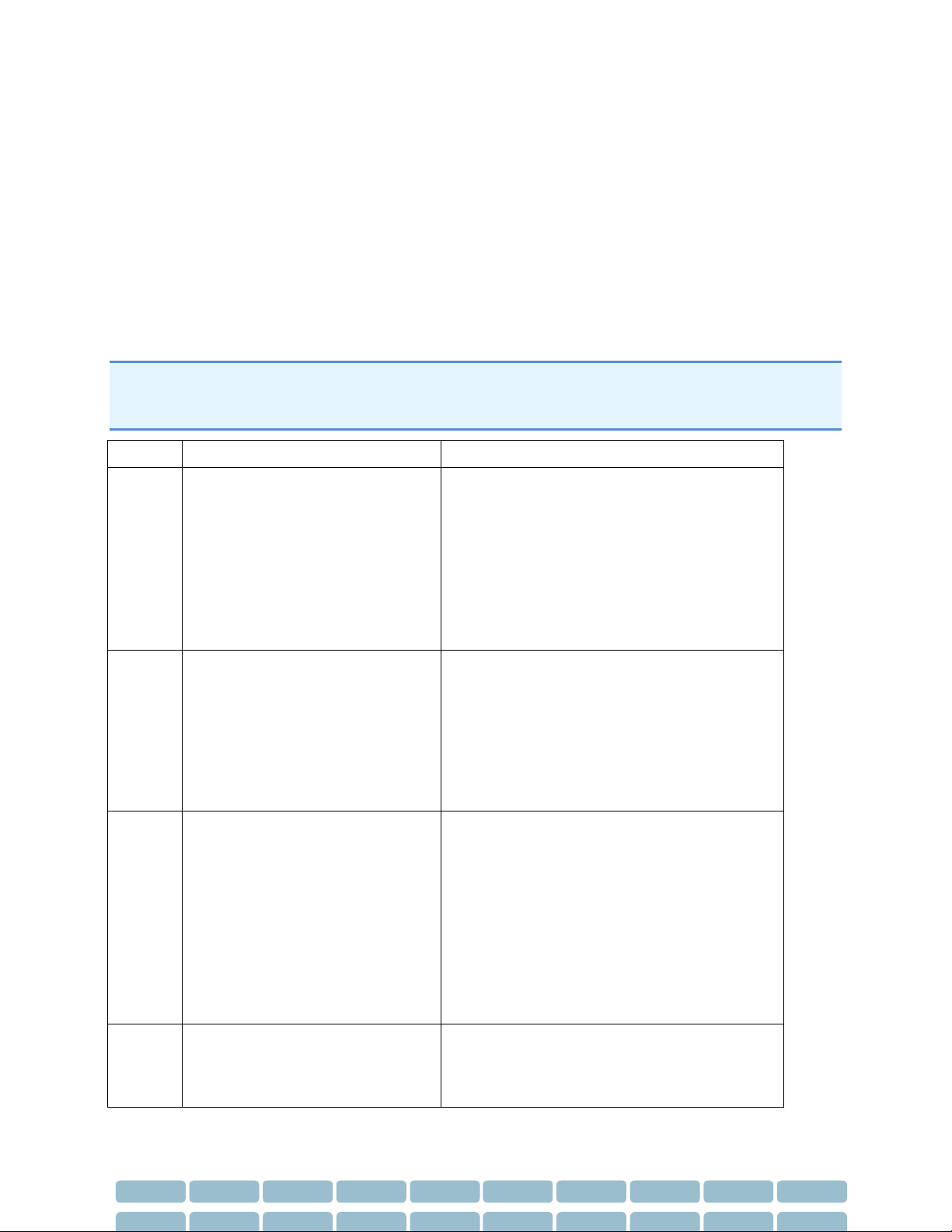
Error Codes
The following is a list of possible errors that may be encountered.
Note: If any error occurred where the code is not listed in the following table, exit XCEL software, turn
OFF/ON the device and then start XCEL software. If problem persists please contact AtCor Medical
Technical support.
Error Code
Error Message
Action
1
Unable to continue Brachial
Calibration due to weak
oscillometric signal (Code = 1).
Ensure use of correct cuff size, correct
positioning and fit
Ensure that no obstructions (eg, clothing)
are located between the cuff and the
patient’s arm
After completing these steps, attempt
another measurement
2
Unable to continue Brachial
Calibration due to erratic
oscillometric signal (Code = 2).
Ensure that the patient’s arm is stationary
during the measurement
Ensure use of correct cuff size, correct
positioning and fit
After completing these steps, attempt
another measurement
3
Unable to continue Brachial
Calibration due to exceeded retry
count (Code = 3).
Ensure that the patient’s arm remains
stationary during the measurement
Ensure use of correct cuff size, correct
positioning and fit
Remove any obstructions between the cuff
and the arm
After completing these steps, attempt another
measurement
4
Unable to continue Brachial
Calibration due to exceeded
measurement time limit (Code = 4).
Before retrying the measurement:
Ensure that the patient’s arm remains
stationary during measurement
Page 7
Error Code
Error Message
Action
Ensure use of correct cuff size, correct
positioning and fit
Remove any obstructions between the cuff
and patient’s arm
After completing the above steps, attempt
another measurement
85
Unable to continue Brachial
Calibration due to pneumatic
blockage (Code = 85).
Ensure that the hose is not bent or pinched
Ensure that the hose and cuff is not under
the patient
Ensure use of correct cuff size, correct
positioning and fit
After completing the above steps, attempt
another measurement
87
Unable to continue Brachial
Calibration due to inflate timeout
(Code = 87).
Ensure that the hose connections to the
device and cuff are intact and tight
Ensure use of correct cuff size, correct
positioning and fit
Ensure that the cuff is not leaking air
After completing the above steps, attempt
another measurement
88
Unable to continue Brachial
Calibration due to safety timeout
(Code = 88).
Turn the device OFF and then turn it ON
Ensure use of correct cuff size, correct
positioning and fit
Ensure that the patient’s arm is stationary
during the measurement
After completing the above steps, attempt
another measurement
89
Unable to continue Brachial
Calibration due to cuff
overpressure (Code = 89).
Ensure use of correct cuff size, correct
positioning and fit
Ensure that the hose is not bent or pinched
Ensure that the cuff and hose are not
beneath patient
After completing the above steps, attempt
another measurement
90*
Unable to continue Brachial
Calibration due to hardware
Turn the device OFF.
Page 8
Error Code
Error Message
Action
problem (Code = 90).
Ensure the data/power input are
connected
Turn the device ON
After completing the above steps, attempt
another measurement
103*
File “DatabaseUpgradeReport.csv”
is in use by other application
(code=103)
Close the application
608*
Unable to backup database to
<pathname> (Code = 608).
Select a different folder for backup
(preferably at the local PC instead of the
network), and then attempt to backup the
database again
609*
Unable to restore database from
<pathname> (Code = 609).
Copy the backup file in a different folder
(preferably at the local PC instead of the
network)
Try to restore from the new location of the
backup file.
611*
Unable to migrate data due to
database error (Code = 611).
Ensure that the PC can locate the SQL data
server.
Restart the SphygmoCor XCEL software.
800*
Database server is not responding
(Code = 800).
Restart the computer
Insert the SphygmoCor XCEL installation
DVD
Select Create Database and follow the
instructions on the screen.
2046
Unable to continue Capture due to
unsuccessful start of capture (Code
= 2046).
Ensure that the device is connected to the
computer.
Ensure the cuff is connected to the device.
Ensure that there aren’t any tubing
obstructions
Turn the device OFF and ON.
Attempt Capture again.
2047
Unable to continue Capture due to
unsuccessful stop of capture (Code
= 2047). "Press the STOP
button on the module to deflate
Ensure that the device is connected to the
computer
Ensure that the cuff is connected to the
device.

Page 9
Error Code
Error Message
Action
the cuff
Ensure use of the correct cuff size and that
it is correctly positioned
Turn the device OFF and ON.
Attempt Capture again.
2049
2050
Unable to continue Capture due to
unsuccessful inflation of cuff (Code
= 2049).
(Code = 2050)
Ensure the device is connected to the
computer.
Ensure the cuff is connected to the device.
Ensure use of the correct cuff size and that
it is correctly positioned
Turn the module OFF and ON.
Attempt Capture again.
2051
2053
Unable to continue Capture due to
unsuccessful deflation of cuff (Code
= 2051)
(Code = 2053)
Press the STOP button on the device to
deflate the cuff.
Ensure that the device is connected to the
computer.
Ensure that the cuff is connected to the
device.
Ensure use of the correct cuff size and that
it is correctly positioned
Turn the device OFF and ON.
Attempt Capture again
2058
Unable to continue Capture due to
exceeding the maximum number of
inflation/deflation cycle per
measurement (Code = 2058).
Ensure that the patient’s limb with cuff is
stationary during measurement.
Ensure use of correct cuff size, correct
positioning and fit.
Ensure that there are no obstructions such
as thick clothing between the cuff and arm.
For PWV mode: Ensure the tonometer is
detecting pulses.
After completing the above steps, attempt
Capture again.
2076
Unable to continue Capture due to
invalid data from the Electronics
Module (Code = 2076).
Press the STOP button on the device.
Ensure that the device is connected to the
computer.
In the Setup screen, click on the System and
Page 10
Error Code
Error Message
Action
select Find Module.
Attempt Capture again.
2077
2078
Unable to continue Capture due to
Communication error
(Code=2077)
(Code=2078)
Press the STOP button on the device.
Turn the device OFF and ON.
Ensure that the device is connected to the
computer.
Exit the XCEL software
Start the XCEL software
In the Setup screen, click on the System
and select Find Module.
Attempt Capture again.
2079
Unable to continue Capture due to
deflation of cuff (Code = 2079).
Ensure that the patient’s limb with cuff is
stationary.
Ensure use of the correct cuff size and that
it is correctly positioned
Ensure that there are no obstructions
between the cuff and arm.
After completing the above steps, attempt
Capture again.
2080
Unable to continue Brachial
Calibration due to brachial pressure
being outside the normal range
(Code=2080)
Ensure systolic pressure is within the range
50 to 260 mmHg and diastolic pressure is
within the range 40 to 200 mmHg.
Ensure use of correct cuff size, correct
positioning and fit
Ensure that there are no obstructions
between the cuff and arm.
After completing the above steps, repeat
measurement.
2081*
Unable to continue Brachial
Calibration due to timeout.
Turn the module OFF/ON
Click OK button
Proceed with the measurement
2090*
One of these messages related
to “mismatch serial number”
error will appear:
In the Set Up screen, click System Key
Enter the correct system key provided by AtCor
Medical
Page 11
Error Code
Error Message
Action
(a) Unable to find the
Electronics Module due to
mismatch serial number
(b) Unable to start Brachial
calibration due to
mismatch serial number
(c) Unable to start Capture
due to mismatch serial
number
2091
One of these messages related
to “incomplete calibration”
error will appear:
(a) Unable to find the
Electronics Module due to
incomplete calibration
(b) Unable to start Brachial
calibration due to
incomplete calibration
(c) Unable to start Capture
due to incomplete
calibration
Close SphygmoCor XCEL Software
XCEL Electronics Module need to be calibrated.
Please refer to SphygmoCor XCEL Calibration
KIT or contact AtCor Medical for technical
support.
3051*
Unable to generate PWV Report
(Code= 3051).
Ensure that the entered distance in mm is
correct.
Ensure use of the correct cuff size and that
it is correctly positioned
Ensure waveform quality (use the guidance
bar).
After completing the above steps, repeat
PWV measurement
3052*
Unable to generate PWV Report
(Code= 3052).
Ensure there is enough memory in the
computer.
Ensure the user has read/write permission
for the computer on which the SphygmoCor
XCEL software installed
Ensure the entered distance in mm is
correct.
Ensure use of the correct cuff size and that

Page 12
Error Code
Error Message
Action
it is correctly positioned
Ensure waveform quality (use the guidance
bar).
After completing the above steps, repeat
PWV measurement
3053*
Unable to generate PWA Report
(Code= 3053).
Ensure use of correct cuff size, correct
positioning and fit
Ensure waveform quality (use the guidance
bar).
After completing the above steps, repeat
PWA measurement.
3054*
Unable to generate PWA Report
(Code= 3054).
Ensure there is enough memory in the
computer.
Ensure that the user has read/write
permission where the XCEL software
installed.
Ensure use of correct cuff size, correct
positioning and fit
Ensure waveform quality (use the guidance
bar).
After completing the above steps, repeat
PWA measurement.
3060
Unable to start Capture (Code=
3060).
Ensure that the device is connected to the
computer.
Ensure that the cuff is connected to the
device.
Ensure use of correct cuff size, correct
positioning and fit
Turn the device OFF and ON.
After completing the above steps, attempt
Capture again.
3101*
Unable to complete the operation
due to system error (Code=3101)
Close SphygmoCor XCEL software
Turn the device OFF and ON.
Open SphygmoCor XCEL software
3125
Unable to calculate PWV (Code=
3125).
Ensure that the entered distance in mm is
correct.

Page 13
Error Code
Error Message
Action
3126*
Unable to find the Electronics
Module (Code = 3126).
Ensure the device is on.
Ensure the device is connected to the
computer.
In the Setup screen, click System and select
Find Module.
3136*
Unable to continue due to the
incompatible database version
(code=3136)
Upgrade SphygmoCor XCEL software
*If the problem persist persists, please contact AtCor Medical Technical support.
Database Manager
Database Manager allows you to backup and restore databases. You should backup the database
regularly to protect against the possibility of losing important patient data. To access the Database
Manager, click Database at the top of the screen.
Backup a Database
1. Select Database - Backup.

Page 14
2. Choose a location to save your back up file and click Save.
The backup database must not be saved in any of the following locations:
C:\
C:\Program Files
C:\Documents and Settings
Desktop
My Documents
Any network folder
Restore a Database
The Database –Restore feature is used if a database becomes corrupted or needs to be installed onto
another PC.
Note: When the SphygmoCor XCEL is used by multiple users, SphygmoCor XCEL should not be accessed
by other users before the database is restored.
1. Select Database - Restore.

Page 15
2. Click Yes if you are certain that you want to replace your existing database with a backup database.
Navigate to the location of the backup file and click Open. The restore will be completed
automatically.
Note: When restoring the SphygmoCor XCEL database from an earlier version , a message will appear
indicating that database upgrade report is generated and the earlier version XCEL database is backed up.

Page 16
Basic System Care
Stability
Place the SphygmoCor XCEL device gently onto a stable surface. Dropping the unit can cause damage
and result in the unit not operating correctly.
Choose a proper location that is free of clutter and away from high activity areas where the device could
be accidentally dropped.
Pressure or impact damage
Do not apply heavy pressure to the SphygmoCor XCEL device or subject it to strong impact. Excessive
pressure or impact can damage electronic components or otherwise cause malfunctions.
Temperature
The SphygmoCor XCEL System should be used and stored in ambient temperature and relative humidity
within the range outlined in Technical Specifications in the user manual.
Caution: Do not expose the unit to dirt, moisture or dust. Exposing the unit to dust or moisture could
cause it to fail. Do not place the unit in direct sunlight –prolonged sun or heat exposure may result in
overheating and damage to internal components. If you place the unit near a window, leave it inside
your car, or take it outside in direct sun, it may overheat and damage internal components.
Magnetic fields
Magnets, television sets, radios, large electric motors or any other source of strong magnetic fields such
as MRI devices will affect the operation or may cause it to fail.
Caution: Do not place any components near strong magnetic fields.
Liquids
Liquids on or inside any components of the SphygmoCor XCEL System can cause irreversible damage.
Caution: Do not spill liquids on any component.
Weight
Weight applied to the top of the SphygmoCor XCEL device may cause the unit enclosure to crack and
other parts to be damaged.
Caution: Do not place any objects on top of the SphygmoCor XCEL device. Items on top of the
SphygmoCor XCEL device may obscure or inadvertently activate the Stop Button.
Movement
Sudden jolts can cause damage to the unit or the tonometer if it isn't stored properly.
Caution: Do not shake or drop the unit.

Page 17
Shutting down
Do not power the PC off until the SphygmoCor XCEL software and Windows software have been exited.
Wait 4 seconds after turning the computer OFF before turning it on again.
Caution: Exit the SphygmoCor XCEL software prior to shutting down the computer.
Equipment Calibration
The SphygmoCor XCEL System should be checked annually for calibration. For repairs, refer to qualified
service personnel as instructed by AtCor Medical. The device does not contain any user-serviceable or
reusable parts. Disassembly of the device by unauthorized personnel voids any warranty conditions.
Cleaning Instructions
To clean the SphygmoCor XCEL device, first unplug it from the computer and the power outlet. Using a
damp cloth with mild detergent, gently wipe the equipment. Do not use other cleaning agents. Ensure
excess liquids are wiped immediately from the equipment.
Caution: Do not spray cleaning agents or liquid directly on any components.
Notebook Batteries
Ensure when using the SphygmoCor XCEL System on notebook computers running on rechargeable
batteries that the batteries are fully charged. Do not use the system on low battery power. If the
notebook is abruptly shuts down, the SphygmoCor database may be corrupted. Consult the notebook
manufacturer’s user documentation regarding the safety and maintenance of the notebook
rechargeable battery.
Tonometer Care
The tip of the tonometer is a delicate and sensitive device, and can be easily damaged if dropped or
misused. Follow the guidelines below to ensure tonometer lifetime is maintained.
When the tonometer is not in direct use with the patient, protect the tonometer by placing it in the
storage holder in the tray or in the temporary storage holder on top of the SphygmoCor XCEL device.
Do not use this tonometer with any other instrumentation other than that supplied by AtCor Medical.
Do not use a tonometer from any other supplier. The SphygmoCor XCEL tonometer is intended to be
used in conjunction with the AtCor Medical SphygmoCor XCEL device only, which has a floating
(isolated) grounding system.
Disinfection Instructions for Tonometer and Cuffs
The SphygmoCor XCEL product is considered a “non-critical” device. Therefore a low-level disinfection
method has been provided to assist users to disinfect the tonometer and cuffs, which are the only
patient contacting component of the SphygmoCor XCEL System (see below).
For tonometer disinfection, use a 70% Isopropyl Alcohol (IPA) impregnated wipe or cloth for low-level
disinfection. Allow a contact time of at least 5 minutes.
For cuff cleaning, refer to the cleaning instructions accompanied in the cuff packaging.

Page 18
Caution: Do not immerse the tonometer in any liquid as this will damage the tonometer electronics. Do
not use coarse cloths for wiping the tonometer as this will damage the sensitivity of the transducer.
PWV Thigh Cuff Care
Cleaning Cuffs: Removable covers on straight and contoured cuffs make cleaning easy. The cuff covers
are made of Nylon and Velcro. Remove the bladder then wash the cuff cover in a washing machine on
gentle cycle or by hand using mild soap. Open the top of the cuff and line dry only. When completely
dry, reinsert the bladder. If bladder is contaminated, wash it in soapy water and rinse well without
getting any liquid in the bladder or tubing.
Disinfecting Cuffs: Spray or wipe entire surfaces of cuff with disinfectant until wet. Allow the cuff to
remain visibly wet for a minimum of 10 minutes to insure complete disinfection. Wipe dry with clean
cloth. The following disinfectants have been tested for compatibility with Hokanson cuffs. The
manufacturers do not claim effective disinfection on porous surfaces; we have been unable to find any
disinfectants that claim disinfection on porous surfaces. Hydrogen peroxide, hydrogen peroxide with
silver (Sanosil®), hydrogen peroxide, peroxyacetic acid, silver (Steriplex°"), silver with citric acid
(PureGreen24Tm & SpectraSanTM 24), Octyl decyl dimethyl ammonium chloride with dioctyl dimethyl
ammonium chloride with didecyl dimethyl ammonium chloride with dimethyl benzyl ammonium
chloride (ProtexTM), 99% isopropyl alcohol, or T-SprayTM. Note: Some disinfectants may cause the cuff
blue color to bleed into cuff labeling. This does not affect the cuffs performance.
WARRANTY: Cuffs are guaranteed for one year from the date of purchase.
PWA Brachial Cuff Care
Follow the application instructions for use to ensure the correct size cuff for the patient. Failure to
do so will adversely affect the accuracy of the reading.
Avoid contact with the cuff, other than that of the patient's limb, while measurement is in progress.
Do not compress or pinch cuff tubing.
Promptly remove cuff from patient when monitoring is not in progress
During frequent or extended measurement, check cuff site and limb to ensure proper blood flow. Do
not apply cuff where circulation may be compromised.
Cleaning instructions
The following cleaning methods have been applied 20 times to the cuff without any apparent negative
effects.
The cuff may be sprayed with a mild disinfectant solution (e.g. Cidezyme® ENZOL®, or 10% bleach
solution), rinsed with distilled water and line dry. Ensure that no liquid enters tubing.
OR
To machine wash the cuff, remove the bladder and fully engage the hook and the loop. Machine
wash warm with a mild detergent (50 -130° F or 1 - 54° C) and line dry.

Page 19
Calibrating SphygmoCor XCEL
Warning: The SphygmoCor XCEL System requires annual calibration. Failure to do so may result in the
device being out of specification. This may result in inaccurate measurements.
When calibration is due, the following message will appear in the SphygmoCor XCEL application:
Note: The SphygmoCor XCEL device will continue to take measurements even though the calibration
period has expired.
2 options are available for completing calibration:
1- Contact your distributor or AtCor Medical to arrange for your SphygmoCor XCEL to be returned
for calibration.
2- Purchase a Calibration Kit. Contact your distributor or AtCor Medical for further information.
SphygmoCor XCEL Calibration Kit Operating Instructions
Intended Use
The SphygmoCor XCEL Calibration Kit calibrates the SphygmoCor XCEL Device by providing a rigid 1/2L
volume and a pumping bulb to pressurise the pneumatic system. An external calibrated manometer
(electronic manometer, Sphygmomanometer mercury column, or anaerobic Sphygmomanometer) with
indication in mmHg and resolution of 1mmHg shall be used with the SphygmoCor XCEL Calibration Kit.
The SphygmoCor XCEL Calibration Kit is intended to be used by medical professionals and biomedical
service personnel.
Warning: The aneroid Sphygmomanometer or manometer that will be used to calibrate the
SphygmoCor XCEL, must be calibrated prior to calibrating the SphygmoCor XCEL device.
Basic Care
As per the relevant areas of the Basic System Care section for the SphygmoCor XCEL.
Note: If the following message appears in SphygmoCor XCEL application indicating incomplete or
unsuccessful calibration, then refer to the Restarting the Calibration Procedure Section in the Calibration
Troubleshooting section.

Page 20
WARRANTY: The SphygmoCor® XCEL Calibration Kit components are guaranteed for one year from the
date of purchase
Calibration Kit Components
The Calibration Kit contains the following components:
1- Calibration Pneumatic kit
2- 1/8 ” male to 1/8” male adapter
3- 3/16” male to 3/16” male adapter
4- 3/16” female to 1/8” male adapter
Other manuals for XCEL
1
Table of contents
Popular Medical Equipment manuals by other brands

Henry Schein
Henry Schein 900-8275 Instructions for use
Gima
Gima Rescue LIFE user manual

MicroPort
MicroPort SpiderView user manual

Advanced Instruments
Advanced Instruments Osmo1 Service guide

3RAY Electronics
3RAY Electronics ECG-2301G Operation manual

Sizewise
Sizewise Bari Rehab Platform 3 Series user manual