SpiroClinic Pro User manual

User Manual
Welcome to SpiroClinic Pro
Before using your SpiroClinic Pro device and mobile application, please ensure that you
have read this user manual, all labeling and information provided with the product. The
user manual can be downloaded and/or printed from Inofab Health website and Apps.
13513.03
SCP-TF-18 R03
2/123

INDEX
INDEX 4
Introduction 7
Product Description 7
What’s in the box? 7
Intended Use 8
Restrictions on use and Contradictions 9
Parameters 11
Operation 13
Operating Environment 13
Setting up the Device 14
Handpiece 14
Dock 17
Airway 20
Bacterial Viral Filter (BVF) 21
Application 23
Device Indicators 24
Handpiece 24
Dock 25
Performing a Lung Function Test 26
General Method Performing a Spirometry Test with SpiroClinic Pro: 26
Types of Breathing Maneuvers 32
Expiration-Only (Ex-Only) Test Breathing Maneuver: 32
Full Loop Test Breathing Maneuver: 34
The Maximum Voluntary Ventilation (MVV) Test Breathing Maneuver: 36
The Slow Vital Capacity (SVC) Test Breathing Maneuver: 36
Understanding the Test Quality 37
Signs and Symbols 39
Technical Features 41
Safety Warnings and Precautions 42
Maintenance 45
Calibration-Check 45
SCP-TF-18 R03
3/123

Preparation of Calibration Check 46
Cleaning and Disinfection 47
Batteries 49
Instructions for Handpiece Battery Replacement 49
Instructions for Dock Battery Replacement 53
Disposal of SpiroClinic Pro 56
Troubleshooting 56
Orderable Accessories 59
Terms of Warranty 59
Electromagnetic Compatibility 60
Manufacturer Information 64
SpiroClinic Pro 66
SCP-TF-18 R03
4/123

1. Introduction
1.1. Product Description
SpiroClinic Pro is a spirometer that pairs (via Bluetooth®) and operates with smart devices
running iOS, Android, or Windows. SpiroClinic Pro measures and displays certain parameters of
lung function of the user. The user performs a spirometry test as described in the Performing A
Lung Function Test section of this user manual.
Briefly, as the user exhales or inhales through the device airway, internal ultrasonic sensors
detect the velocity of the exhaled/inhaled air, the device converts this information into
spirometric data and displays it on the SpiroClinic Application. The SpiroClinic App prompts and
guides the user throughout the test for accurate data collection and registration. The app can be
downloaded on Apple’s App Store, Google Play Store, Huawei App Gallery or Microsoft Store.
SpiroClinic Pro consists of a handpiece, a removable airway(SpiroWay Pro), and a dock
station(SpiroClinic Dock).
Dock Station (SpiroClinic Dock) automatically captures ambient conditions and gives the
information directly to SpiroClinic Application. Users review this information and can change or
approve the ambient conditions.
The device is powered by 2 x AA batteries for the handpiece and 2 X AA batteries for the dock.
SpiroClinic Pro works with the SpiroWay Pro Airway and a conventional bacterial viral
filter(BVF)..
1.2. What’s in the box?
The SpiroClinic Pro box contains:
● SpiroClinic Pro Handpiece
● SpiroClinic Pro Dock
● SpiroWay Pro Airway
● Mini screwdriver
SCP-TF-18 R03
5/123
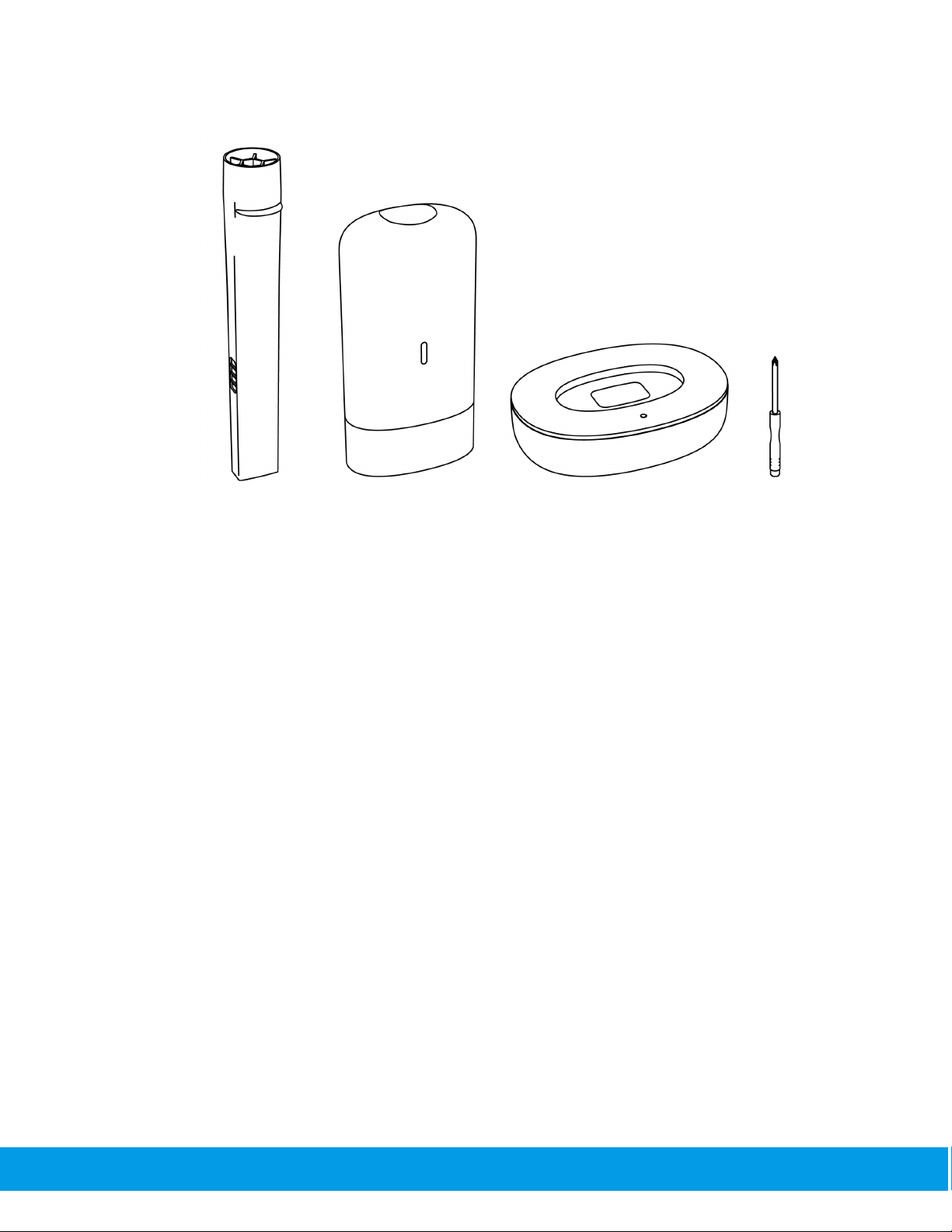
Figure 1: SpiroClinic Pro Box Content.
Caution: Please check to ensure that there is no visible damage on any of the components of
the SpiroClinic Pro. If the damage is present, do not use or attempt to repair the device, please
contact the manufacturer directly.
1.3. Intended Use
SpiroClinic Pro is intended to be used as a professional spirometer for lung function testing. See
the Parameters section for more information on measured parameters. The SpiroClinic Pro is
indicated for:
- children (over the age of 5), adolescents or adults who may have been diagnosed with a
chronic pulmonary disease including, but not limited to, asthma, chronic obstructive
pulmonary disease and cystic fibrosis.
and should be used by:
- healthcare professionals such as lab technicians, physicians, nurses, occupational
health professionals etc.
SCP-TF-18 R03
6/123

1.4. Restrictions on use and Contradictions
Any diagnosis of conditions or prescribed treatments should be made only by a qualified
healthcare professional. The healthcare professional should take into consideration the
outcomes of a medical examination, the patient’s clinical history and results of any other tests
deemed necessary, in addition to the test results provided by SpiroClinic Pro.
SpiroClinic Pro is a multi-user device. The device can log the information and test results that
belong to each specific patient. For each new patient, a new patient account must be created on
the SpiroClinic App, so that each user's personal information and test results can be stored and
logged.
A new disposable bacterial viral filter must be used for each new user.
The spirometry test should only be performed by users who do not experience any shortness of
breath and are in good health for performing a lung function test. Test results of patients who do
not meet these conditions may not be reliable. A correct spirometry test depends greatly on the
patient’s ability to correctly perform the expiratory/inspiratory maneuver as described in this
manual. Failure to perform a correct maneuver may lead to inaccurate and unacceptable
results. The device should not be used if the accuracy and reliability of test results may be
jeopardized by external factors.
Performing spirometry can be physically demanding. The forced expiratory maneuver used in
spirometry increases intrathoracic, intraabdominal, and intracranial pressures. Potential risks of
spirometry are primarily related to maximal pressures generated in the thorax and their impact
on abdominal and thoracic organs, venous return and systemic blood pressure, and expansion
of the chest wall and lung. The physical effort required can increase myocardial demand.
Caution must be used for patients with medical conditions that could be adversely affected by
these physiological consequences. Although such risks are likely to be minimal for spirometry in
most patients, the potential risks associated with testing should always be weighed against the
benefit of obtaining information about lung function. Spirometry should be discontinued if the
patient experiences pain during the maneuver. Patients with potential contraindications that
would prevent testing in the primary care setting may be tested in a pulmonary function
laboratory where operators are more experienced and there may be access to emergency care
if needed. Furthermore, because spirometry requires the active participation of the patient,
inability to understand directions or unwillingness to follow the directions of the operator will
usually lead to submaximal test results.
Relative Contraindications for Spirometry;
Due to increases in myocardial demand or changes in blood pressure;
SCP-TF-18 R03
7/123

➢Acute myocardial infarction within 1 wk
➢Systemic hypotension or severe hypertension
➢Significant atrial/ventricular arrhythmia
➢Non-compensated heart failure
➢Uncontrolled pulmonary hypertension
➢Acute cor pulmonale
➢Clinically unstable pulmonary embolism
➢History of syncope related to forced expiration/cough
Due to increases in intracranial/intraocular pressure;
➢Cerebral aneurysm
➢Brain surgery within 4 wk
➢Recent concussion with continuing symptoms
➢Eye surgery within 1 wk
Due to increases in sinus and middle ear pressures;
➢Sinus surgery or middle ear surgery or infection within 1 wk
Due to increases in intrathoracic and intra abdominal pressure;
➢Presence of pneumothorax
➢Thoracic surgery within 4 wk
➢Abdominal surgery within 4 wk
➢Late-term pregnancy
Infection control issues;
➢Active or suspected transmissible respiratory or systemic infection, including tuberculosis
➢Physical conditions predisposing to the transmission of infections, such as hemoptysis,
significant secretions, or oral lesions or oral bleeding
Please ask the patient if they have or suspect having any of the conditions above before use of
the Spiro Clinic Pro.
SCP-TF-18 R03
8/123
1.5. Parameters
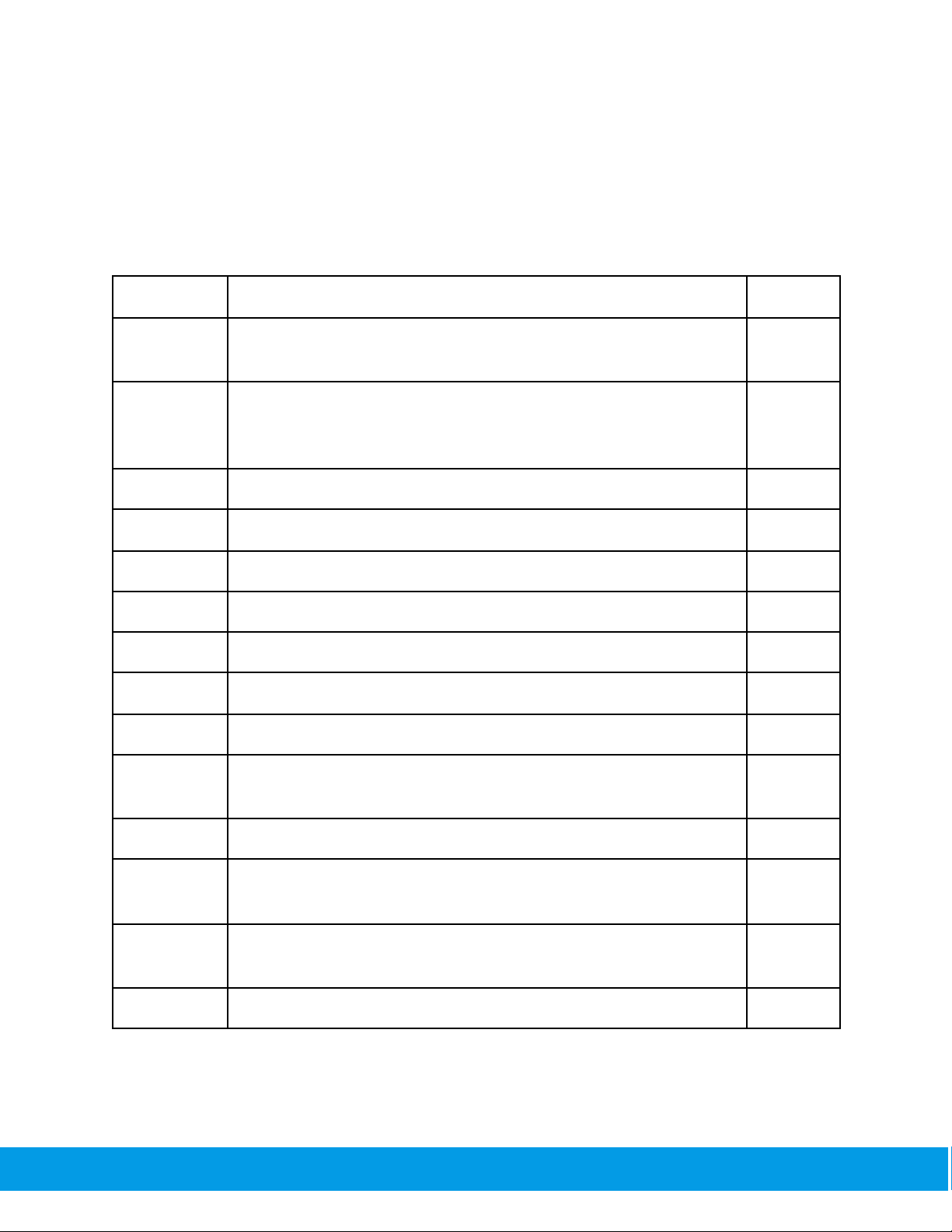
The SpiroClinic Pro records and displays the following spirometry data:
Parameters
Definition
Unit
FVC
Forced Vital Capacity — The volume of air that can forcibly be
blown out after taml inspiration
L
FEV0.75
Forced Expiratory Volume within 0.75 seconds: The volume of air
that can forcibly be blown out within 0.75 seconds, after taml
inspiration.
L
FEV1
Forced Expiratory Volume within 1 second
L
FEV3
Forced Expiratory Volume within 3 seconds
L
FEV6
Forced Expiratory Volume within 6 seconds
L
FEV0.75/FVC
The ratio of FEV0.75 to FVC
--
FEV1/FVC
The ratio of FEV1to FVC
--
FEV3/FVC
The ratio of FEV3to FVC
--
FEV6/FVC
The ratio of FEV6to FVC
--
PEF
Peak Expiratory Flow — The maximal flow rate achieved during
the maximally forced expiration initiated at taml inspiration.
L/s
MMEF
Mean Mid-Expiratory Flow — synonymous with FEF25-75
L/s
FEF25
Forced Expiratory Flow at 25% of vital capacity — synonymous
with MEF75
L/s
FEF50
Forced Expiratory Flow at 50% of vital capacity — synonymous
with MEF50
L/s
FEF75
Forced Expiratory Flow at 75% of vital capacity —synonymous
L/s
SCP-TF-18 R03
9/123
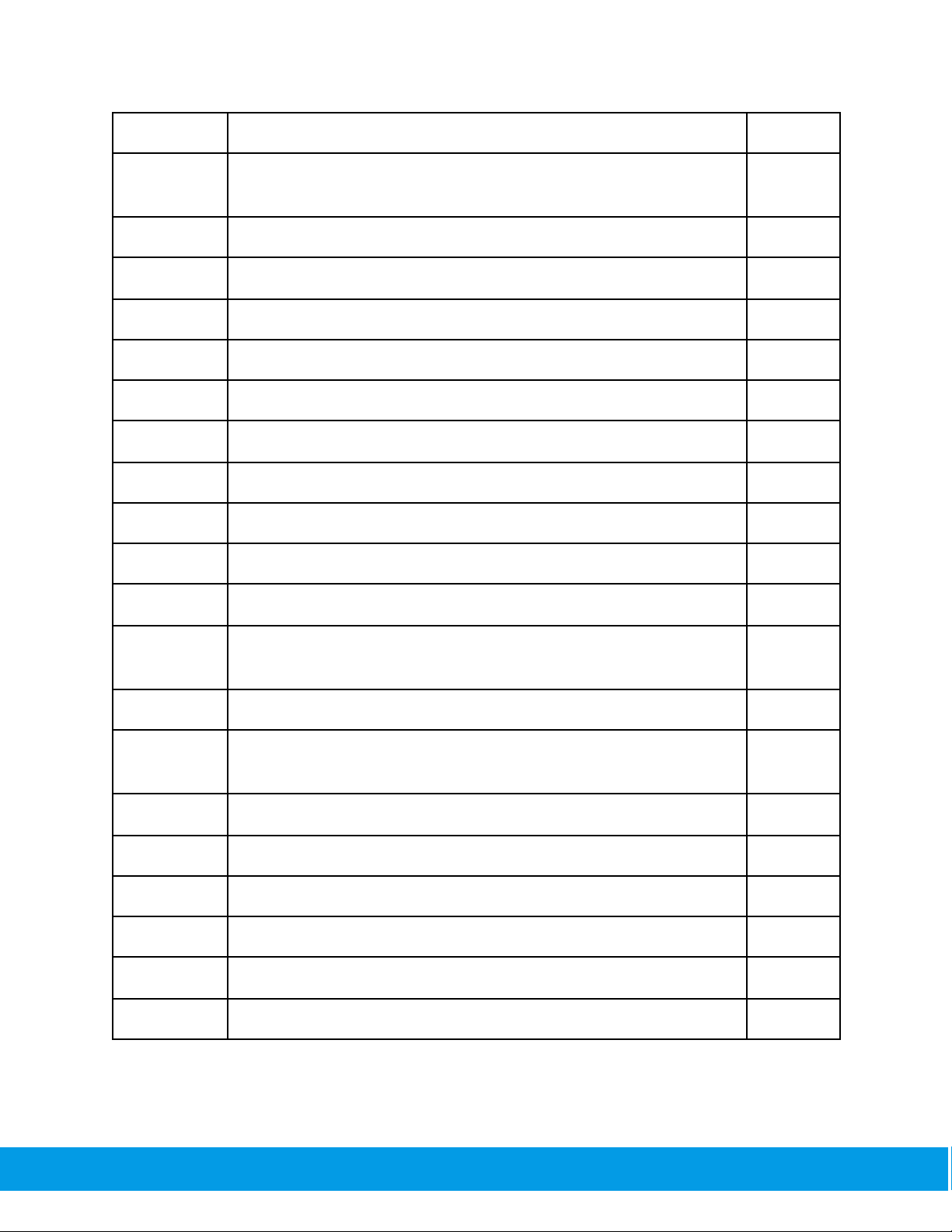
with MEF25
FEF25-75
Forced Expiratory Flow from 25% to 75% of vital capacity —
synonymous with MMEF
L/s
MET25-75
Mıd-Expıratory Tıme — synonymous with FET25-75
s
FEV0.75/FEV6
The ratio of FEV0.75 to FEV6
--
FEV1/FEV6
The ratio of FEV1to FEV6
--
FEF50/FVC
The ratio of FEF50 to FVC
1/s
MMEF/FVC
The ratio of MMEF to FVC
1/s
FET
Forced Expiratory Time
s
BEV
Back extrapolated volume
L
FIV1
The forced inspiratory volume within 1 second
L
FIVC
Forced inspiratory vital capacity
L
PIF
Peak inspiratory flow
L/s
FIF25-75
Forced inspiratory flow at 25% of vital capacity — synonymous
with MIF75
L/s
FIV1/FIVC
The ratio of FIV1to FIVC
--
R50
(FEF50/FIF50)
The ratio of flow at 50% of expiration and flow at 50% of
inspiration — synonymous with FEF50/FIF50
--
VC
Vital capacity, from slow expiration
L
VCin
Inspiratory vital capacity, from slow inspiration
L
VCex
Expiratory vital capacity, from slow expiration
L
ERV
Expiratory reserve volume
L
IRV
Inspiratory reserve volume
L
IC
Inspiratory capacity from end of tidal breathing
L
SCP-TF-18 R03
10/123
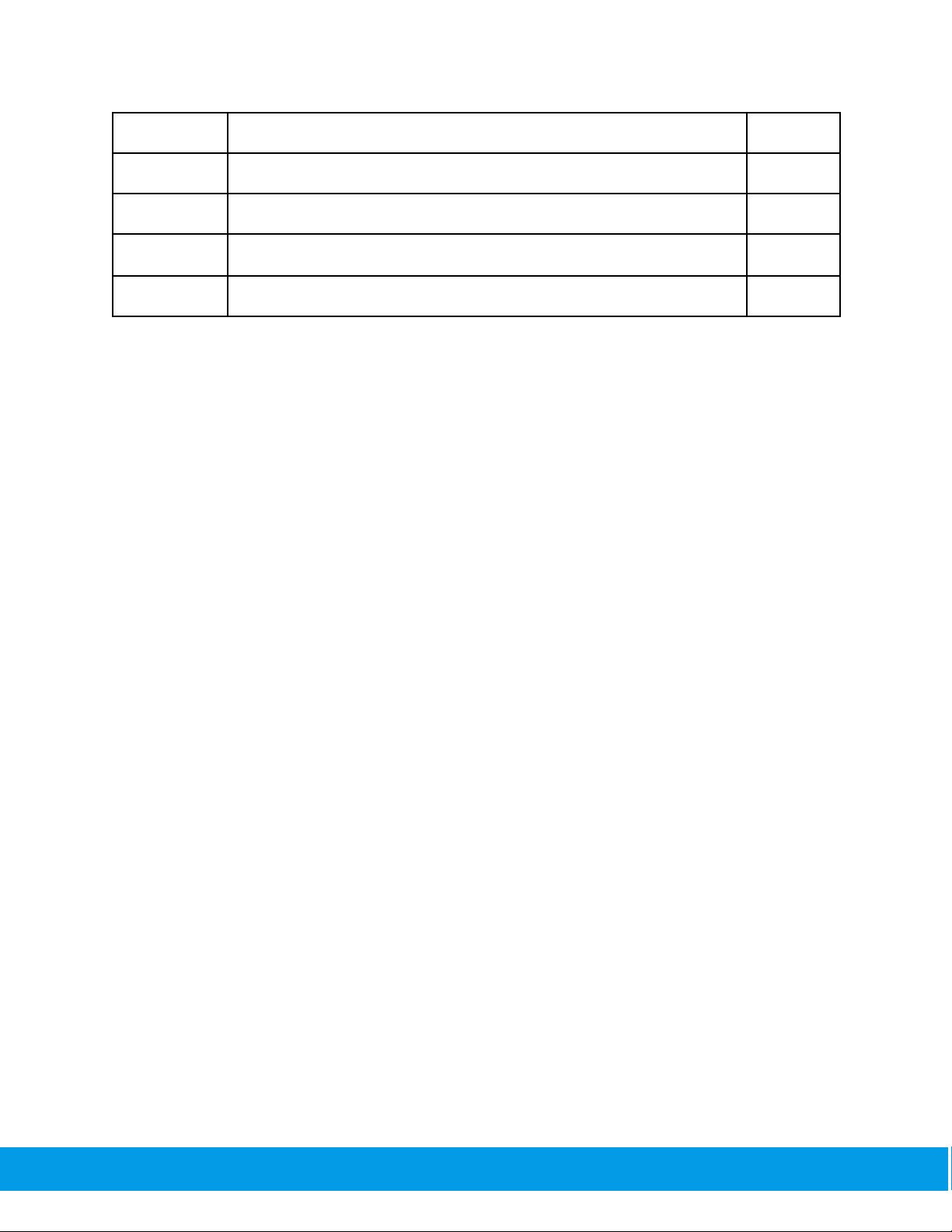
Rf
Respiratory frequency
1/min
VT
Tidal Volume
L
MVV
Maximum voluntary ventilation
L/min
MVV6
Maximum plat voluntary ventilation for 6 seconds
L/min
MVVtime
Duration of the trial in seconds
s
The recommended number of trials per spirometry session is 3, however, the user may perform
up to 8 trials. The best values obtained from the spirometry tests performed in one session are
displayed on the app interface. Users and healthcare professionals have the option to view the
results of each spirometry trial performed in a spirometry session separately.
The device also provides a reference value (obtained from large epidemiological studies on the
patient’s height, weight, sex and ethnicity). Test results from spirometry tests are compared to
the reference value and displayed as a percent predictive value indicator of the patient’s
respiratory health. The patient’s personal best value for a spirometry session should be
discussed with them for medical interpretation.
Caution: Interpretation of spirometry results or diagnosis of any medical conditions must only
be made by a qualified physician or allied health care professional experienced in spirometry.
2. Operation
2.1. Operating Environment
The SpiroClinic Pro is designed for use in a clinical setting by multiple users.
The operating conditions for the SpiroClinic Pro are specified as:
Temperature: +15°C to +35°C
Relative Humidity: 10% to 85%
The storage conditions for the SpiroClinic Pro are specified as:
SCP-TF-18 R03
11/123

Temperature: -20°C to +50°C
Relative Humidity: 0% to 90%
Pressure: 500 hPa to 1060 hPa
SpiroClinic Pro should not be used in rapidly changing environmental conditions even if the
conditions are in the recommended range.
SpiroClinic Pro should not be used in the presence of flammable liquids or detergents, nor in the
presence of inflammable anaesthetic gases (oxygen or nitrogen).
The device should not be used in direct air currents (e.g. wind), sources of heat or cold, direct
sun rays or other sources of light or energy, dust, sand or any other chemical substances.
The Device should not be used with extremely high RF noise in the environment.
The Device Should not be used near the sources of strong electromagnetic radiation or sources
of ultrasonic sound.
SpiroClinic Pro and its Dock should be in the range of bluetooth connection to the smart device
they are connected to.
2.2. Setting up the Device
2.2.1. Handpiece
Remove the battery cover by unscrewing it with the screwdriver provided with the device.
SCP-TF-18 R03
12/123
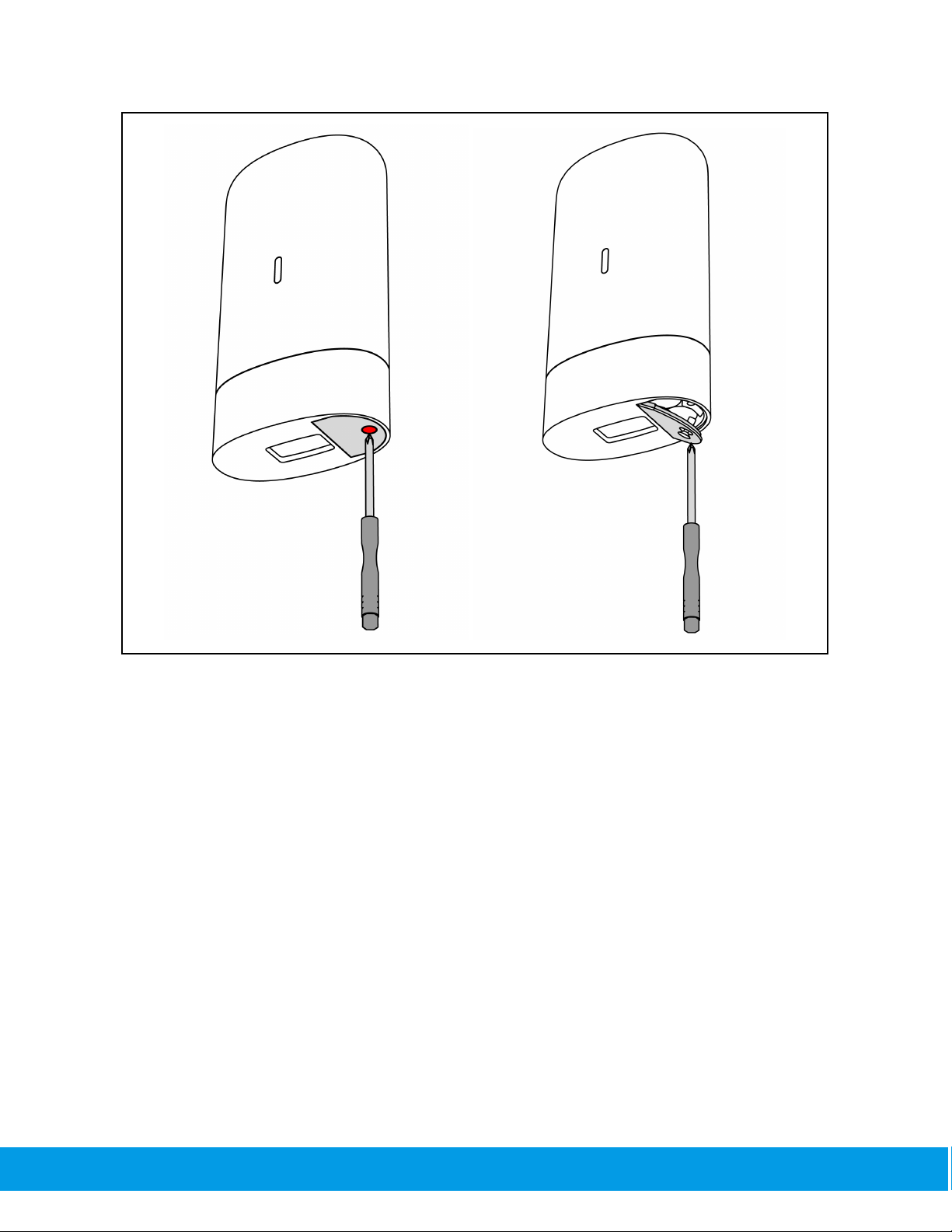
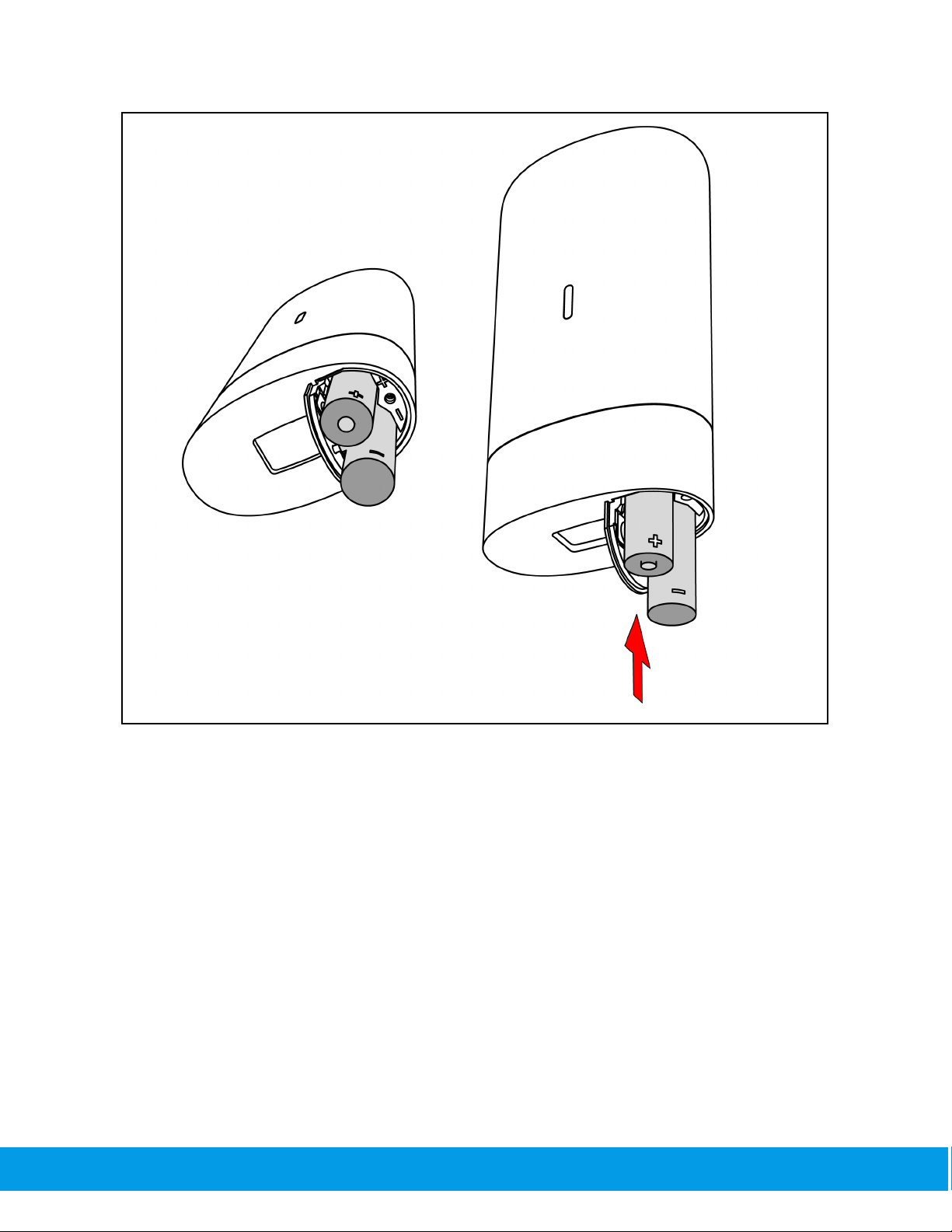
Place the AA batteries in the correct orientation, screw the battery cover back to the closed
position.
SCP-TF-18 R03
13/123
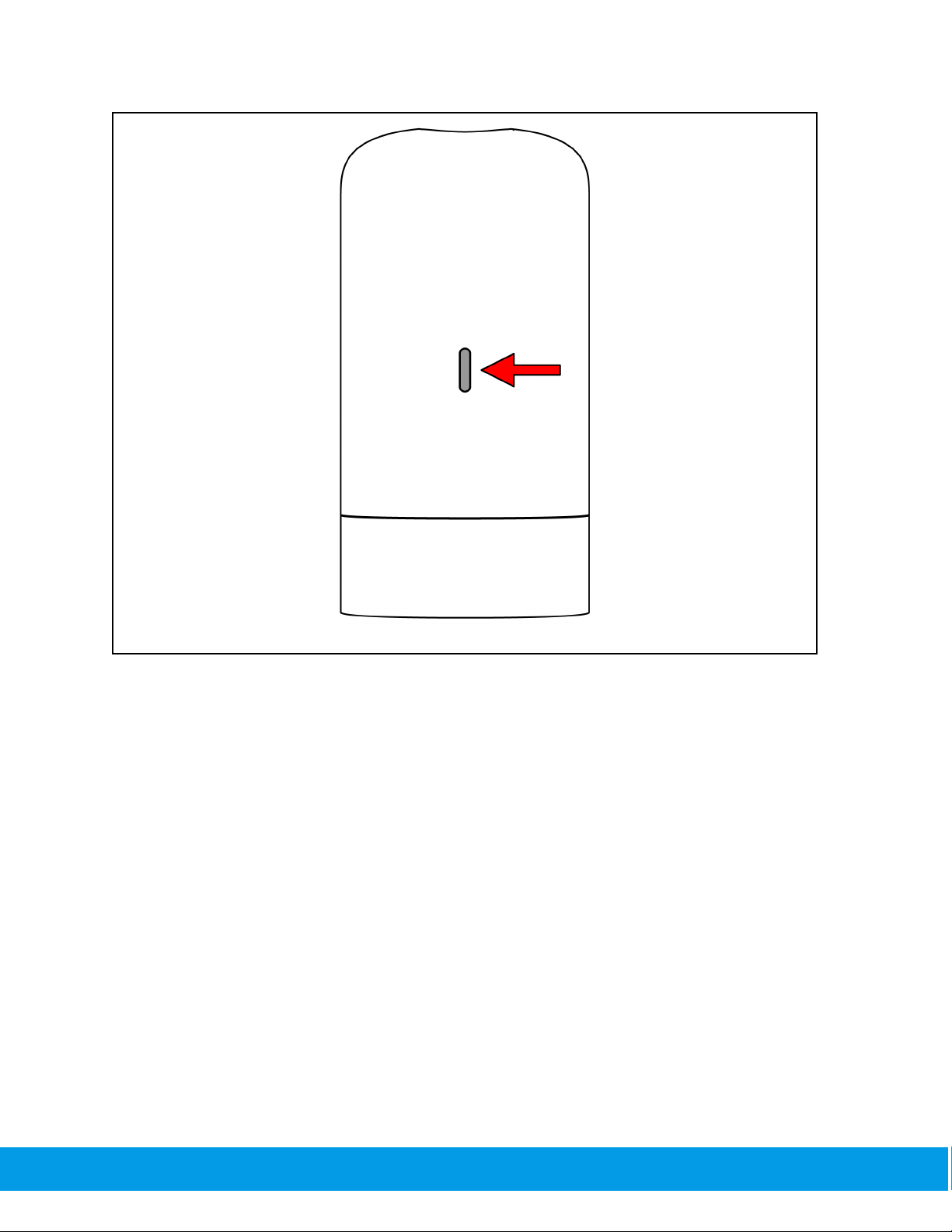
The handpiece should give an indication light and be ready to use.
SCP-TF-18 R03
14/123
2.2.2. Dock
Remove the battery cover by unscrewing it with the screwdriver provided with the device.
SCP-TF-18 R03
15/123

Place the AA batteries in the correct orientation, screw the battery cover back to the
closed position.
SCP-TF-18 R03
16/123
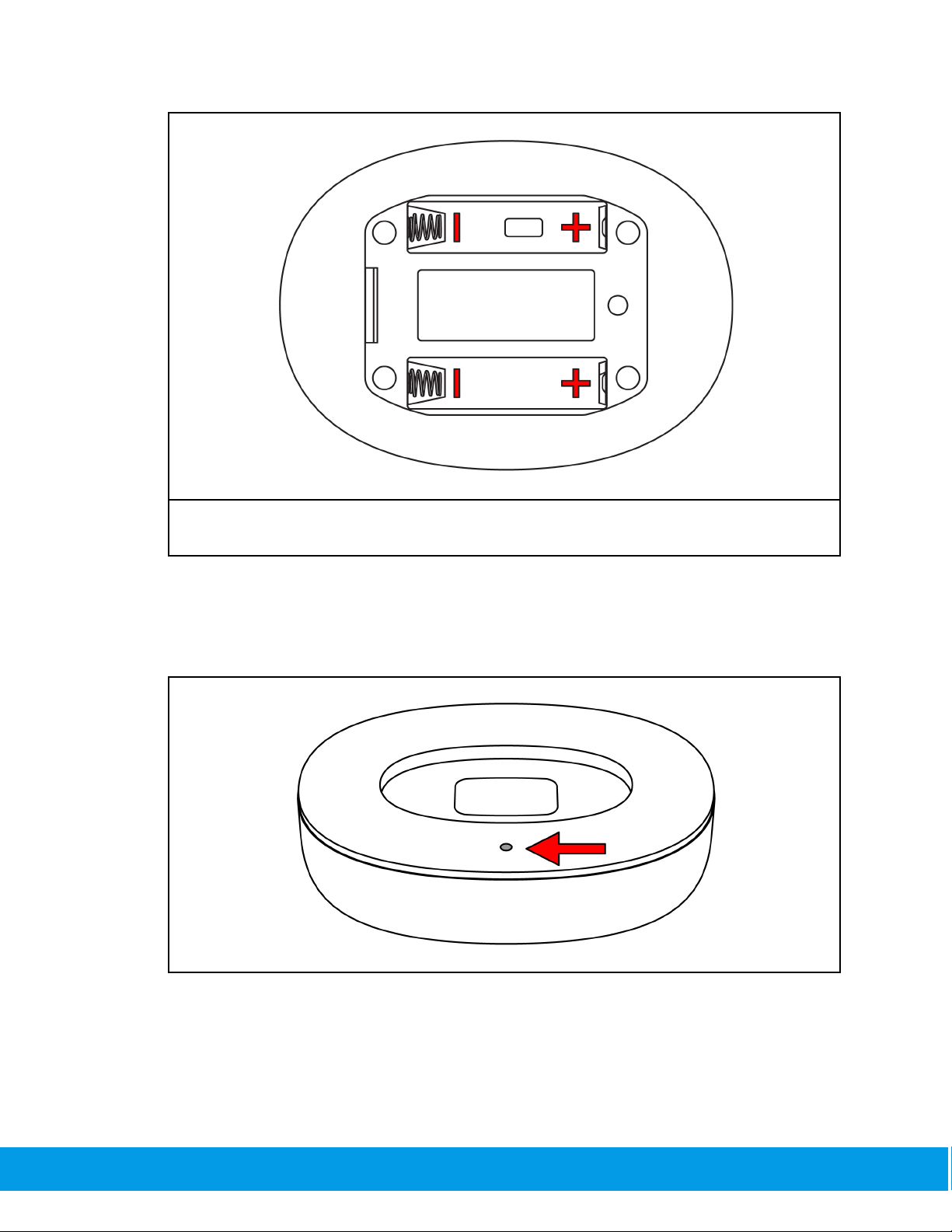
The dock should give an indication light and be ready to use.
SCP-TF-18 R03
17/123
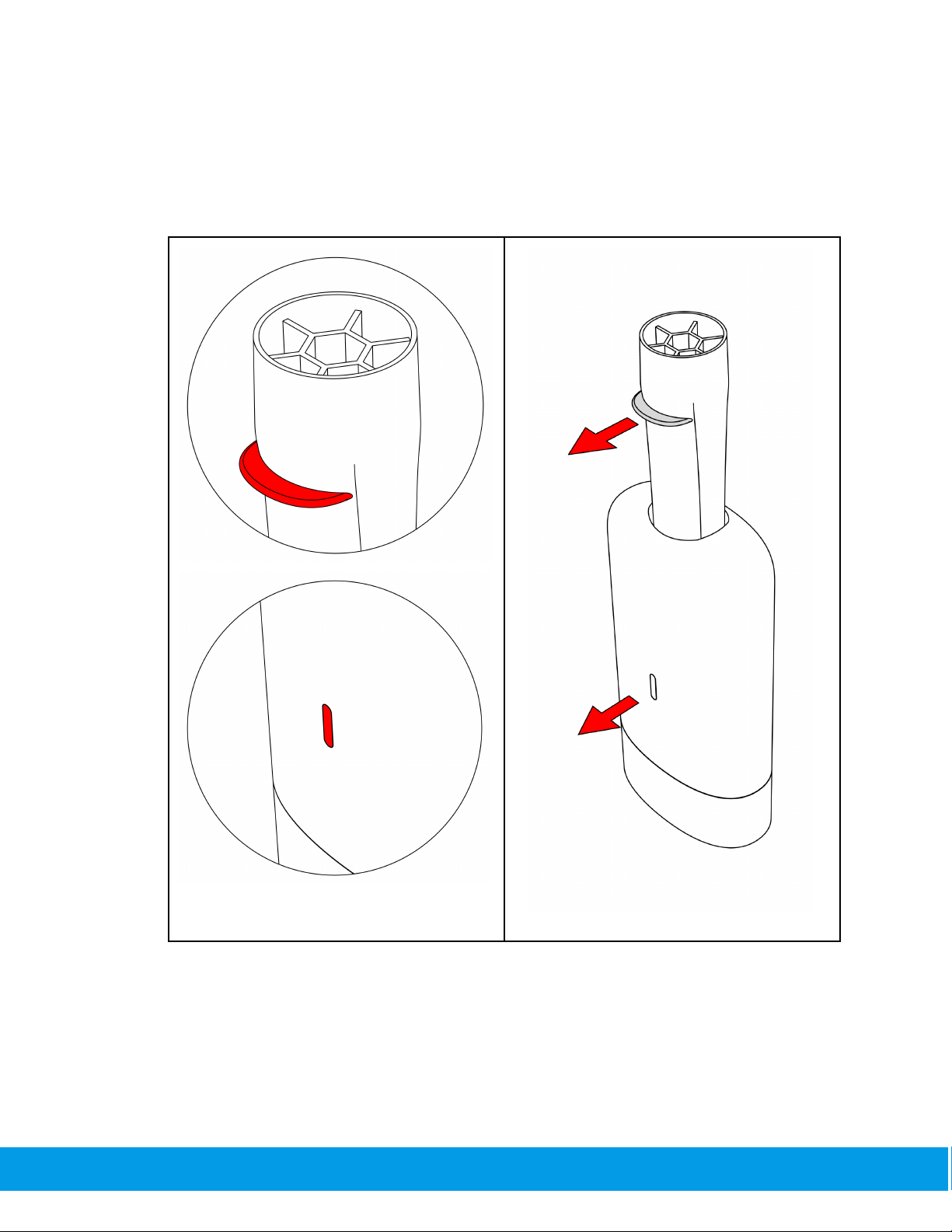
2.2.3. Airway
Insert the SpiroWay Pro to the body with the handle forward.
SCP-TF-18 R03
18/123
2.2.4. Bacterial Viral Filter (BVF)
Attach Bacterial Viral filter (BVF) to the SpiroWay Pro which is attached to the SpiroClinic
SCP-TF-18 R03
19/123

Pro and make sure it is fit and sealed.
Do not use SpiroClinic without BVF. Do not use BVF more than once, and adhere to
user instructions of the used BVF.
Not all BVFs will make a sealed fit to SpiroWay Pro. Furthermore, some BVFs may not
have the required low resistance, quality, or repeatability to ensure accurate
measurements and effective protection against cross-contamination.
Use the Bacterial Viral Filters with the technical specifications provided below:
Resistance: 0-80 kPa*(s/l)
Inner Diameter: 30mm
Please only use the BVFs which complies to the specifications provided by the
manufacturer.
SCP-TF-18 R03
20/123
2.2.5. Application
Download the SpiroClinic App from the App Store, Google Play Store, or Microsoft Store
onto a smart device or PC.
Follow the steps given in the app to create a user account or login to an existing
account.
Enable Bluetooth®on the smart device or PC and pair it with the SpiroClinic Pro by
following the instructions on the app.
SCP-TF-18 R03
21/123
Table of contents
Other SpiroClinic Measuring Instrument manuals
Popular Measuring Instrument manuals by other brands

Andor Technology
Andor Technology Zyla sCMOS 4.2 PLUS Hardware guide

Endress+Hauser
Endress+Hauser Levelflex M FMP41C Safety instructions

LINSHANG
LINSHANG LS220 user manual

Emerson
Emerson Rosemount 8600D Series quick start guide

Extech Instruments
Extech Instruments 445580 user manual

ELIT
ELIT E7II user manual

Geotech
Geotech Biogas 300 operating manual

Dresser
Dresser ROOTS B3 Series Installation operation & maintenance

Sky Control
Sky Control SC8100 Configuration manual

PCB Piezotronics
PCB Piezotronics 339A30 Installation and operating manual

SOMMY
SOMMY SF Series user manual

THORLABS
THORLABS CVH100-COL Quick reference guide






