IFU-3264 Rev B Instructions for Use, Fenom Pro 5
Chapter 1: System Overview
System Description
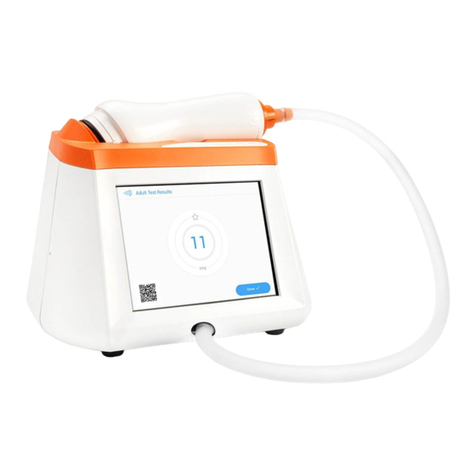
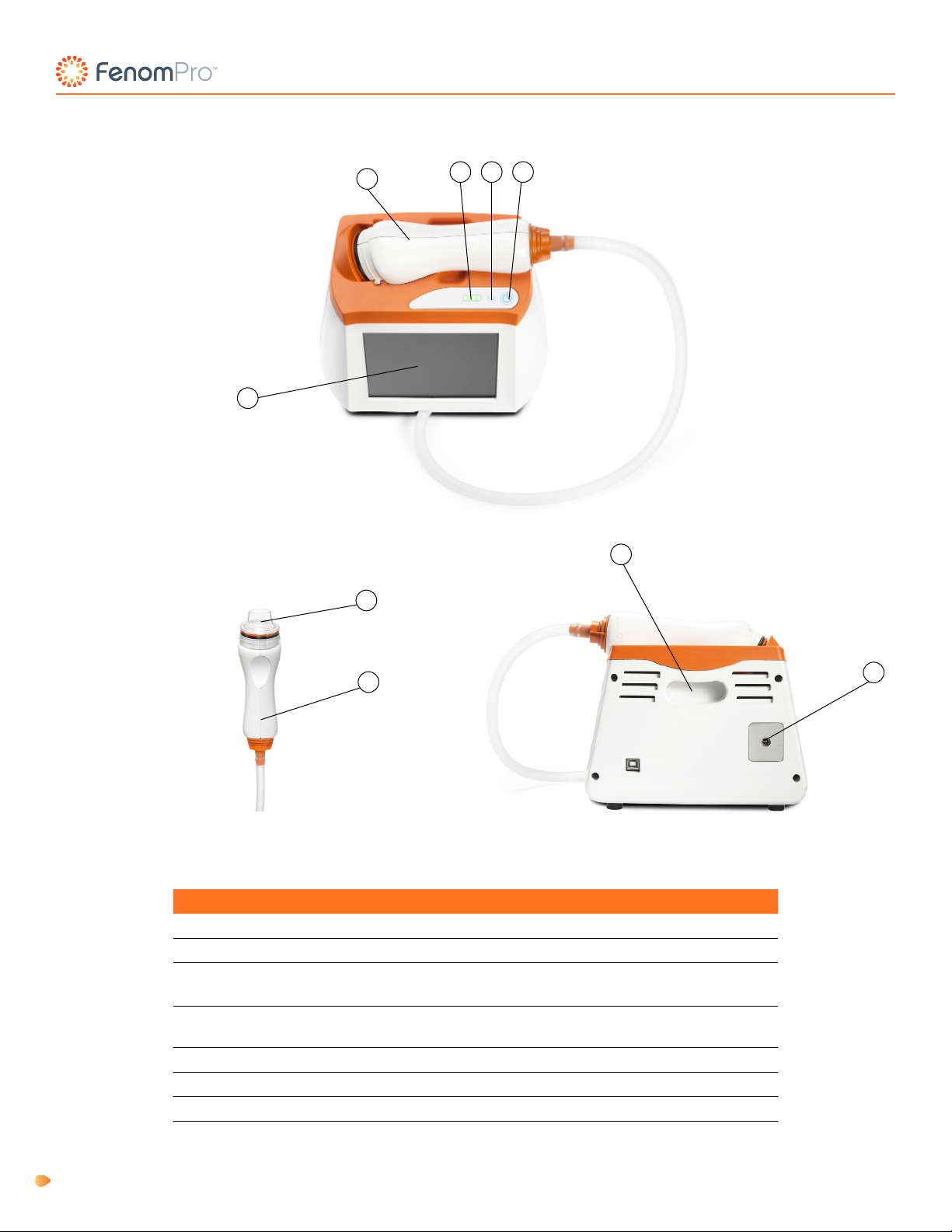
The Fenom Pro™ Asthma Monitor (henceforth known as Fenom Pro) is a point-of-care breath analyser that uses
electrochemical technology to measure the fraction of exhaled nitric oxide (FeNO), a marker for airway inammation, in
human exhaled breath.
Measurement of FeNO by Fenom Pro is a quantitative, non-invasive, simple, and safe method to assess, monitor, and
determine the best treatment methods for airway inammation in patients. The Fenom Pro device is suitable for use in
hospitals and other healthcare settings. The mouthpiece is the applied part for patient use.
Fenom Pro is designed as a hand-held device for measuring FeNO in exhaled breath from humans. The level of
exhaled nitric oxide (NO) is frequently increased in some inammatory processes such as asthma. The fractional
NO concentration in expired breath can be measured by Fenom Pro according to guidelines for NO measurement
established by the American Thoracic Society (ATS) and European Respiratory Society (ERS) [1].
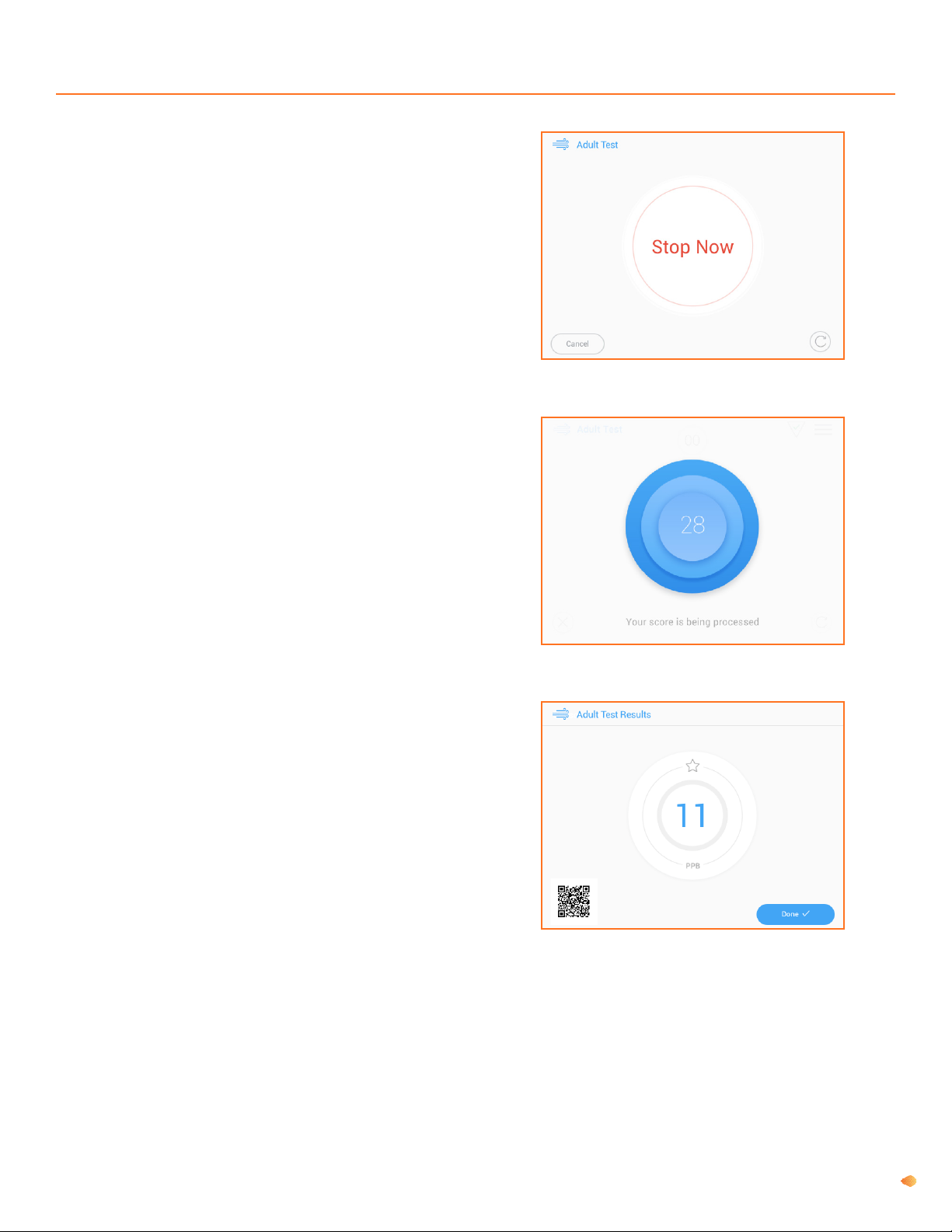
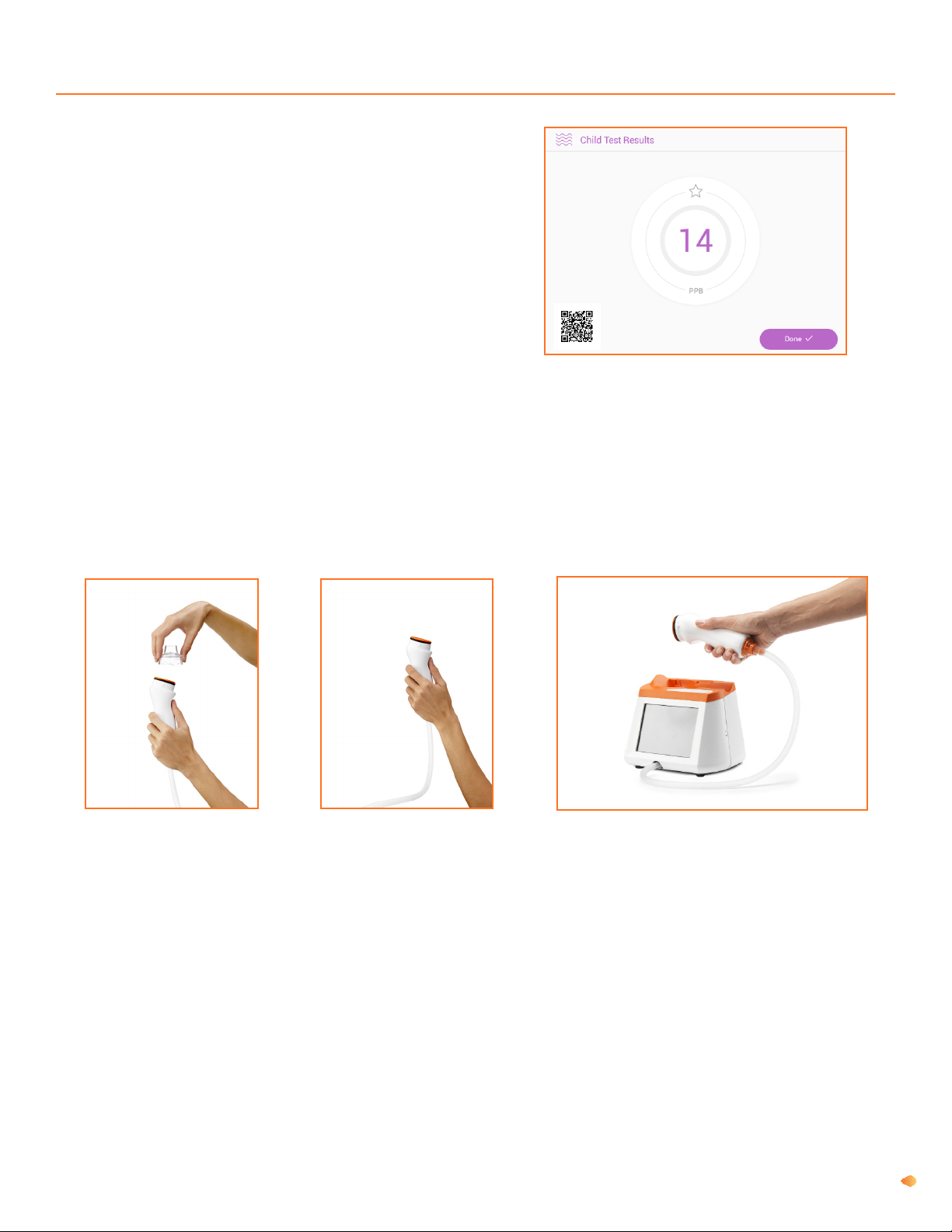
Fenom Pro provides direct sampling with delayed analysis (approximately 28 seconds) of sequentially collected and
analysed exhaled breath. No subsequent specic specimen collection, specimen preparation, or reagents are required.
The emissions characteristics of the Fenom Pro device make it suitable for use in hospitals and other healthcare
settings (CISPR 11 class A).
Indications for Use
Fenom Pro measures FeNO in human breath. FeNO is increased in some airway inammatory processes, such as
asthma, and decreases in response to anti-inammatory treatment [1]. FeNO measurements with Fenom Pro should
be used as part of regular assessment and monitoring of patients with these conditions [10]. Testing using the Fenom
Pro should only be done in a point-of-care healthcare setting under professional supervision. Fenom Pro is suitable for
children, approximately 7-17 years, and adults 18 years and older.
Clinical Limitations
Fenom Pro may not be used by children under the age of approximately 7 years, including infants, as measurement
requires patient cooperation. The determining factor for age limitation is based on a patient’s ability to understand
and execute the given instructions.
Fenom Pro should not be used in critical care, emergency care, or in anaesthesiology.
Elevated FeNO levels are also found in other inammatory conditions aside from asthma, such as allergic rhinitis [2],
systemic lupus erythematosus [3] and liver cirrhosis [4], and COPD including COPD overlap syndrome [5] [6].
Viral infections might lead to increased FeNO levels. The mechanism behind this increase is, however, separate from
the one causing the increased levels seen in allergic inammation. Virus related increases in FeNO may be resistant to
corticosteroid treatment [7].
Recent intake of nitrate rich food, including lettuce, spinach, beets, walnuts, peanuts, and animal organs, can lead to
increased FeNO levels [8].
Smoking reduces exhaled NO levels. However, FeNO can still dierentiate asthmatics from non-asthmatics among
smokers.
Risks to Health
There are no known direct risks to patient health posed by the use of Fenom Pro. However, failure to perform the test
as indicated or erroneous interpretation of results may lead to improper patient management.
Therefore, use of FeNO measurement results to adjust a treatment regimen without consideration of other clinical
factors could pose a risk.