Spirosure FenomPro User manual

IFU-3393 Rev C Instructions for Use, Fenom Pro,
Asthma Monitor
Instructions for Use
R

IFU-3393 Rev C Instructions for Use, Fenom Pro
2
R
Table of Contents
Denitions 4
Chapter 1: System Overview 5
System Description 5
Indications for Use 5
Clinical Limitations 5
Risks to Health 5
Fenom Pro Components 6
Table 1: Fenom Pro Components 6
Display Buttons 7
Table 2: Button and Indication Icons 7
Chapter 2: Safety and Warnings 8
Safety Instructions 8
Compliance 8
Warnings 8
Electromagnetic Emissions 9
Electromagnetic Immunity 9
Chapter 3: Fenom Pro Quick Start Guide 10
Chapter 4: FeNO Measurement Preparation 12
Wake up Device 12
Pre-test Check 12
Chapter 5: Perform FeNO Measurement 13
Perform a FeNO Test 13
Remove Mouthpiece 14
Chapter 6: Quality Control 15
Perform a QC Test 15
QC User Status 15
Create QC Users 15
Chapter 7: Power O Device 16
Chapter 8: Practice Mode 16
Chapter 9: Device Setup 17
Initial Setup 17
Conguration Settings 17
Device Settings 17
Time/Date 17
Language 17

IFU-3393 Rev C Instructions for Use, Fenom Pro 3
System Information 19
Test Incentive Sound 19
Add Tests 19
Quality Control Status 19
Chapter 10: General Care 20
Operating Conditions 20
Cleaning 20
Handling 20
Storage 20
Preventive Inspections 21
Rechargeable Battery 21
Maintenance 21
Disposal of Used/Expired Equipment and Consumables 21
Limited Warranty 21
Chapter 11: Troubleshooting 22
Support 22
Add Tests 22
Error and Codes 23
Table 4: Error Codes 23
Chapter 12: Technical Data 26
Dimensions and Weight 26
Electrical Data 26
Exhaled NO Performance 26
Linearity 26
Precision 26
Accuracy 26
Limit of Detection 26
Measurement Range 26
Exhalation Parameters 26
Chapter 13: Device Performance 27
Accuracy 28
Limit of Detection 28
Chapter 14: Reference 29
Symbol Explanation 29
Chapter 14: Parts and Accessories 30
Parts 30
Accessories 30
Bibliography 31

IFU-3393 Rev C Instructions for Use, Fenom Pro
4
R
Denitions
FeNO Fractional exhaled nitric oxide – Amount of nitric oxide in the exhaled breath originating
from the bronchial passages, not the nasal passages nor upper airway.
FEV1 Forced Expiratory Volume in One Second - Volume of air that can be forcibly exhaled from
the lungs in the rst second of a forced expiratory maneuver, measured in liters.
FEV6 Forced Expiratory Volume in Six Seconds - Volume of air that can be forcibly exhaled from
the lungs in the six seconds of a forced expiratory maneuver, measured in liters.
FVC
Forced Vital Capacity - After the patient has taken in the deepest possible breath, this is
the volume of air that can be forcibly and maximally exhaled out of the lungs until no more
can be expired, usually measured in liters.
NO Nitric oxide – Produced by the human lung and is present in the exhaled breath. It has been
implicated in the pathophysiology of lung diseases, including asthma.
PEF Peak Expiratory Flow - Maximal ow (or speed) achieved during the maximally forced
expiration initiated at full inspiration, measured in liters per minute or in liters per second.
Spirometry Common oce test used to assess how well a patient’s lungs work by measuring how much
air is inhaled, how much is exhaled, and how quickly it is exhaled.
The following are the denitions for terms and abbreviations used in this manual

IFU-3393 Rev C Instructions for Use, Fenom Pro 5
Chapter 1: System Overview
System Description
Spirosure Fenom Pro™ is a point-of-care breath analyzer that uses electrochemical sensor technology to measure the
fraction of exhaled nitric oxide (FeNO), a marker for airway inammation, in human exhaled breath. Measurement of
FeNO by Fenom Pro is quantitative, non-invasive (only the mouthpiece comes in contact with the patient), simple and
safe. Fenom Pro is designed as a hand-held device for measuring FeNO in exhaled breath from humans. The level
of exhaled nitric oxide (NO) is frequently increased in some inammatory processes such as asthma. The fractional
NO concentration in expired breath can be measured by the Fenom Pro™ device according to guidelines for NO
measurement established by the American Thoracic Society (ATS) and European Respiratory Society (ERS) [1].
Fenom Pro™ provides direct sampling with result report within 30 seconds of sequentially collected and analyzed
exhaled air. No subsequent specic specimen collection, specimen preparation, or reagents are required. The
emissions characteristics of the Fenom Pro™ device make it suitable for use in point of care sites and hospitals (CISPR
11 class A).
Indications for Use
Fenom Pro Nitric Oxide Test is a portable, non-invasive device to measure fractional exhaled nitric oxide (FeNO) in
human breath. FeNO is increased in some airway inammatory processes, such as asthma, and often decreases in
response to anti-inammatory treatment. Measurement of FeNO by Fenom Pro is a method to measure the decrease
in FeNO concentration in asthma patients that often occurs after treatment with anti-inammatory pharmacological
therapy as an indication of therapeutic eect in patients with elevated FeNO levels. FeNO measurements are to be
used as an adjunct to established clinical assessments. Fenom Pro is suitable for children, approximately 7-17 years,
and adults 18 years and older.
Testing using the Fenom Pro should only be done in a point-of-care healthcare setting under professional supervision.
Fenom Pro should not be used in critical care, emergency care or in anesthesiology.
Clinical Limitations
Fenom Pro may not be used by children under the age of approximately 7 years, including infants, as measurement
requires patient cooperation. Fenom Pro may not be used by children under the age of 7 years, or by patients who are
unable to understand and execute the instructions given by healthcare providers, as measurement requires patient
cooperation.
Fenom Pro should not be used in critical care, emergency care, or in anesthesiology.
All subjects should refrain from eating or drinking for at least 60 minutes before the FENOM test. Recent intake of
nitrate rich food, such as lettuce, spinach, beets, walnuts, peanuts, and animal organs, can lead to increased FeNO
levels [2].
Smoking reduces exhaled NO levels [5]. Fenom results obtained from subjects who smoke should only be considered
after considering the subject’s smoking history and the potential impact on NO levels.
Risks to Health
There are no known direct risks to patient health posed by use of Fenom Pro. However, failure of the test to perform
as indicated or erroneous interpretation of results may lead to improper patient management.
Therefore, use of FeNO measurement results to adjust a treatment regimen without consideration of other clinical
factors could pose a risk.
IFU-3393 Rev C Instructions for Use, Fenom Pro
6
R
542 3
1
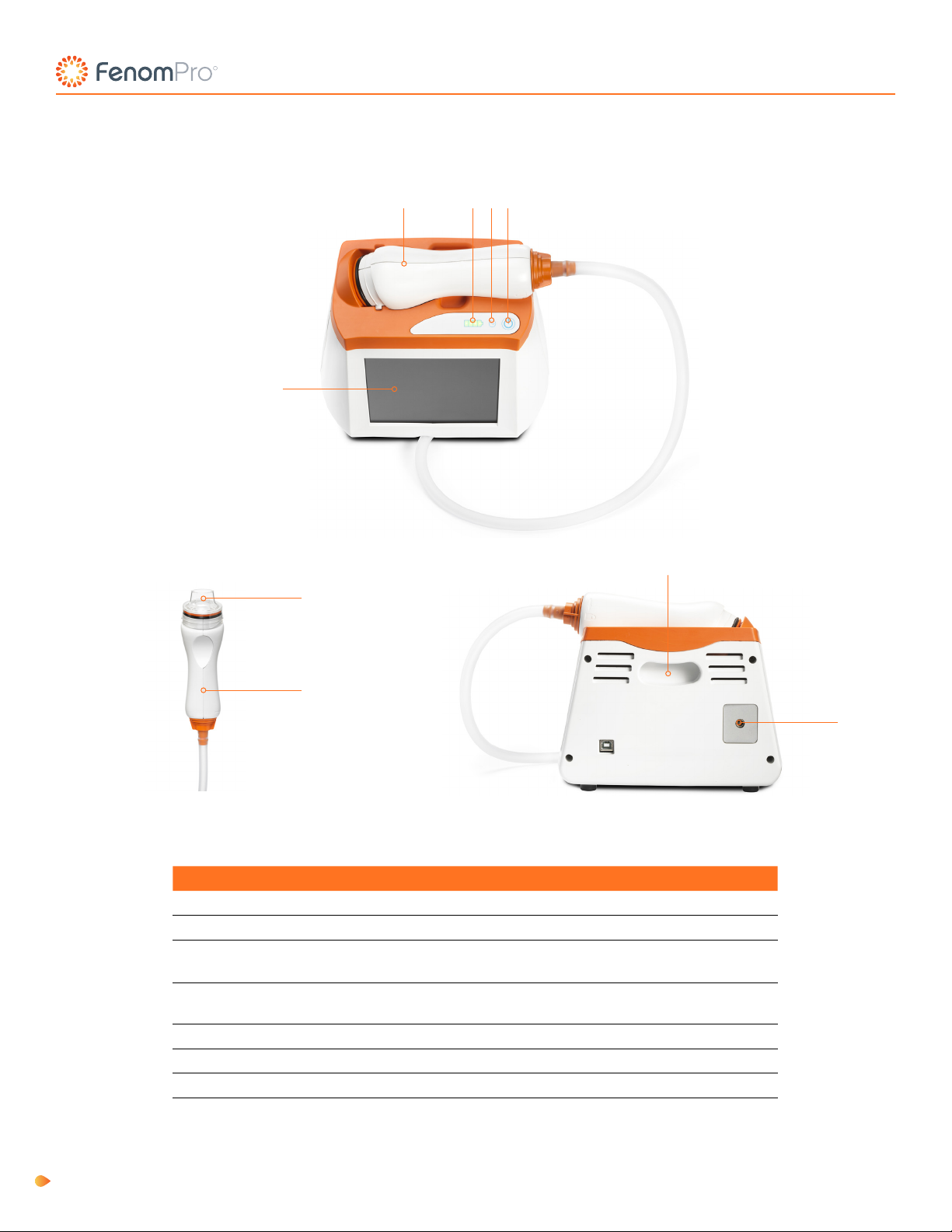
Fenom Pro Components
No. Description
1Touch Screen
2Handpiece
3Battery Indicator –Battery strength is below 25% if only one
bar is illuminated
4AC Power Indicator - Indicator is green when the device is
powered on and connected to an electrical outlet.
5Power Button – Hold for one second to power on/o.
6Single-Patient-Use Mouthpiece (accessory)
7Carrying Handle
824 V Power Connection
Table 1: Fenom Pro Components
7
8
6
2

IFU-3393 Rev C Instructions for Use, Fenom Pro 7
Buttons Icon Name Description
Settings Button
Button used to open the Settings Menu. This menu allows for setting
Time/Date, selecting Language, viewing System Information, selecting
Volume Level, and Ordering Tests.
Test License
Status Button
(Tests available)
Button used to open Order Tests box. Green checkmark indicates that the
device has tests available.
Test License
Status Button
(Few tests available)
Button used to open Order Tests box. Red exclamation point indicates few
tests are remaining. Contact your distributor to order additional tests.
Quality Control Status
Button
(QC Status - Pass)
Button used to indicate current QC Mode Status. Green icon indicates
current QC Mode status is passed.
Quality Control Status
Button
(QC Status - Fail)
Button used to indicate current QC Mode Status. Red icon indicates
current QC Mode status is failed or expired.
Quality Control Status
Button
(QC Status - Disabled)
Button used to indicate current QC Mode Status. Gray icon indicates
current QC Mode status is temporarily disabled or analyzing.
Display Buttons
There are several button icons that Fenom Pro utilizes to help you easily navigate through the menu screens.
Table 2: Button and Indication Icons

IFU-3393 Rev C Instructions for Use, Fenom Pro
8
R
Chapter 2: Safety and Warnings
Safety Instructions
The following safety instructions apply in the handling and operation of Fenom Pro:
oDO NOT inhale through the device.
oDO NOT inhale through the mouthpiece.
oDO NOT exhale beyond the limits of your physical ability.
oDiscontinue measurements if the breath maneuver is laborious for the patient.
oDO NOT allow use of Fenom Pro within 15 minutes after performing spirometry testing such as: FEV1, FEV6,
FVC, PEF, etc.
oDO NOT allow use of Fenom Pro within 60 minutes after exercising or smoking.
oDO NOT use the Fenom Pro device without a new single-patient-use mouthpiece.
oDO NOT perform more than six breath attempts on a single patient within one day.
oIf repeated measurements taken for at least two consecutive days are more than 10 ppb dierent contact
Spirosure, Inc. Technical Support.
oDO NOT bring Fenom Pro in a room containing magnetic resonance equipment.
oDO NOT bring Fenom Pro to a room adjacent to magnetic resonance equipment.
Compliance
Fenom Pro is CE-marked according to the In Vitro Diagnostics Directive 98/79/EC.
Fenom Pro is RoHS compliant according to Directive 2011/65/EU Restriction of Hazardous Substances in Electrical and
Electronic Equipment.
Warnings
The following warnings apply in the handling and operation of Fenom Pro:
oFenom Pro should only be operated by trained healthcare professionals.
oOperate Fenom Pro as stated in this manual. Spirosure accepts no responsibility for damaged equipment or
faulty results if the equipment is not handled according to this manual.
oDO NOT use a damaged Fenom Pro device, damaged components, or damaged accessories.
oUse only power supply unit provided.
oKeep the device out of water. Ensure no liquid is spilled or dripped on the device.
oDO NOT use the Fenom Pro device adjacent to or stacked with other equipment because it could result in
improper operation.

IFU-3393 Rev C Instructions for Use, Fenom Pro 9
Electromagnetic Emissions
The emissions characteristics of this equipment make it suitable for use in hospitals and other healthcare settings
(CISPR 11 class A).
Electromagnetic Immunity
Fenom Pro has been tested to comply with the emission and immunity requirements described in IEC 60601-
1-2:2014 (4th Edition) General requirements for basic safety and essential performance - Collateral standard:
Electromagnetic compatibility - Requirements and tests and AIM 7351731:2017 Medical Electrical Equipment and
System Electromagnetic Immunity Test for RFID Readers.
Fenom Pro is not compatible with magnetic resonance systems, and is labeled as MR Unsafe. The Fenom Pro should
not be used in a room containing a magnetic resonance system or adjacent rooms to a magnetic resonance system.
Note: Sites should ensure that their security systems and other equipment do not interfere with Fenom Pro.
oDO NOT block device vents and ports while in use or while charging.
oDO NOT drop the device or subject it to strong impact.
oNo modication of the Fenom Pro device, handpiece, or mouthpiece is allowed.
oDO NOT use Fenom Pro in the proximity of areas where volatile substances such as organic uids or
disinfectants are being used. Special attention should be paid to aerosols and disinfection baths.
oDO NOT use Fenom Pro in the presence of ammable vapors or liquids
oUse of substances containing alcohol close to the Fenom Pro device may cause erroneous
measurement results. [3]
oSingle-patient-use mouthpiece should be used immediately after opening.
oDO NOT reuse the single-patient-use mouthpiece on other patients.
oWhen not in use, the Fenom Pro device should be stored in the packaging provided.
(See Chapter 12: General Care.)
oDO NOT open, crush, heat above 140 °F/60 °C, or incinerate the lithium-ion battery in the device.
IFU-3393 Rev C Instructions for Use, Fenom Pro
10
R
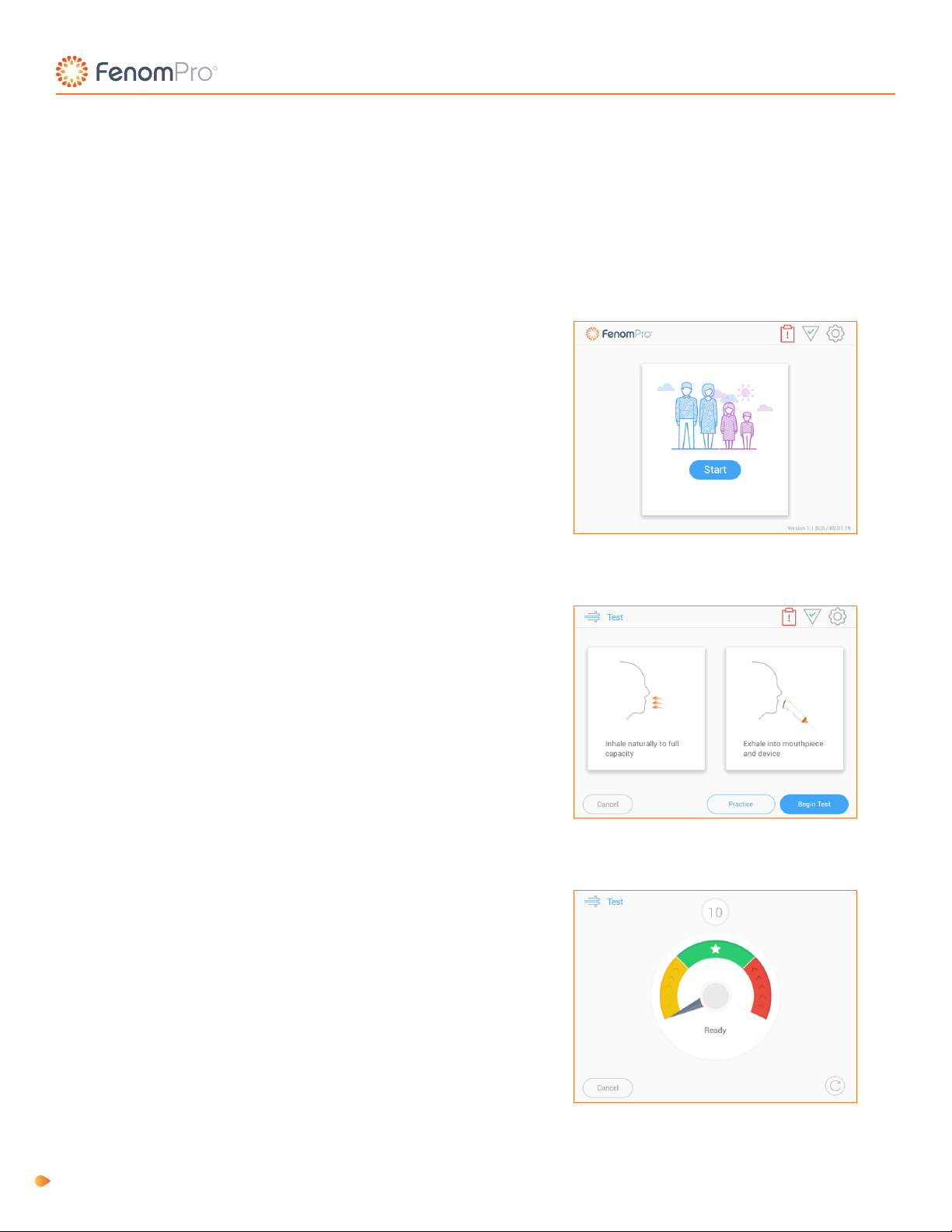
Chapter 3: Fenom Pro Quick Start Guide
To perform a FeNO test, follow these three simple steps. For full test guidelines and instructions, see Chapters 4 and 5.
For setup instructions, see Chapter 9.
NOTE: Check to make sure the device is powered on. If the device is on but the display is blank, touch the screen to
wake up the device. The device may take one minute to warm up.
2. Touch the Begin Test button and instruct the patient
to inhale naturally to full capacity, place mouth on
the mouthpiece ensuring to keep tight seal so no air
escapes and exhale for the full breath test duration at a
steady ow.
1. Select Start test on the Home Screen. Remove a new
single-patient-use mouthpiece from its packaging and
attach it to the hand-piece by pressing the mouthpiece
towards the top of the hand-piece and twist clockwise
to secure. Be careful to not touch the part of the
mouthpiece that will have patient contact.
Instruct the patient to keep the indicator over the star
at the top of the gauge.
NOTE: See Chapter 8: Practice Mode if patient requires
a demonstration before taking the test.
NOTE: Having the indicator within the green range is
also acceptable.

IFU-3393 Rev C Instructions for Use, Fenom Pro 11
3. The Fenom Pro will display the Stop Now screen and
play an audible chime once the patient has successfully
completed the breath maneuver.
Results will display in 28 seconds.
4. Press the Done button and properly dispose of the
used mouthpiece.

IFU-3393 Rev C Instructions for Use, Fenom Pro
12
R
Chapter 4: FeNO Measurement Preparation
NOTE: See Chapter 2: Safety and Warnings for list of safety instructions and warnings.
Pre-test Check
1. Check the battery indicator to ensure the unit has sucient battery power to perform a FeNO measurement.
If the battery indicator is below 25%, plug device into the power supply before using.
2. Check that device is on a at, stable surface while performing a FeNO measurement.
3. Conrm that the patient meets eligibility requirements:
oAge 7 and up.
oHas not consumed food or uids other than water in the preceding 60 minutes.
oHas not exercised or smoked in the preceding 60 minutes.
4. When pre-test check is complete, proceed with Chapter 5: Perform FeNO Measurement.
Wake up Device
1. If the device is powered o, press the Power button to
turn it on.
NOTE: If the device is powered on but the display is
blank, touch the screen to wake up the device.
Allow the device to warm-up for one minute.

IFU-3393 Rev C Instructions for Use, Fenom Pro 13
Chapter 5: Perform FeNO Measurement
The FeNO measurement is performed by the patient blowing into a single-patient-use mouthpiece that is attached to
a handpiece. The patient must blow into the mouthpiece at a controlled rate, which is monitored through an animated
graphic on the touch display. Once a sucient amount of the patient’s breath is captured, the sensor analyzes the
breath and reports a FeNO score in parts per billion (ppb).
Perform a FeNO Test
NOTE: Complete the steps in Chapter 4: FeNO Measurement Preparation before continuing with the steps below.
1. Lift the handpiece out of the cradle on top of the Fenom Pro.
2. Remove the new, single-patient-use mouthpiece from its packaging without touching the part that will go into the
patient’s mouth.
3. Attach the mouthpiece to the handpiece by rmly grasping the outer diameter of the mouthpiece and pushing
towards the top of the handpiece while twisting clockwise until secure.
4. Hand the handpiece to the patient with the mouthpiece attached.
5. Press the Start button on the test selection screen.
6. Provide the patient with a brief overview on how to use the Fenom Pro device.
oInstruct patient to inhale naturally to full capacity before placing mouth
on the mouthpiece.
oInstruct patient to place mouth on the mouthpiece and exhale for a
full 10 seconds at a steady ow.
oInstruct patient to keep lips sealed around the mouthpiece so no breath
escapes from patient’s lips.
NOTE: See Chapter 8: Practice Mode if patient requires a demonstration before
taking the test.
7. Touch the Begin Test button when the patient understands the instructions and is ready to begin.
8. The visual incentive gauge displays.
9. Instruct the patient to begin exhaling into the mouthpiece whenever ready.
10. Ensure that the patient stops exhaling once the Stop Now screen is displayed.
11. If the patient was unsuccessful in performing a breath maneuver, review the reason for the failure. If necessary,
patient can attempt a test in Practice Mode (Chapter 8) before repeating.
12. Proceed to instructions in View Results section of this Chapter.

IFU-3393 Rev C Instructions for Use, Fenom Pro
14
R
Remove Mouthpiece
When the patient has completed performing a FeNO measurement:
1. Remove the mouthpiece by rmly grasping around the outer diameter and twist counter-clockwise while pulling
away from the handpiece.
2. Properly dispose of the used mouthpiece.
3. Replace the handpiece in its cradle on top of the device.
View Results
Upon completion of the FeNO test, the patient’s breath
is analyzed, and the results are displayed in ppb. It takes
approximately 28 seconds for the results to display.
1. View the FeNO result. If the result is less than 10 ppb, “<
10” will be displayed. If the result is greater than
200 ppb, “>200 ppb” will be displayed.
2. Touch the Done button.

IFU-3393 Rev C Instructions for Use, Fenom Pro 15
Chapter 6: Quality Control
The quality control (QC) measurement is performed on a Fenom Pro device by qualied operators. QC Mode is
designed to ensure the instrument is operating within its specications. QC consists of two test types: a Negative
Control, and a User Control. The Negative Control test begins with a standard breath maneuver, but analyzes ambient
air that has been scrubbed of Nitric Oxide. The User Control test is performed by a Qualied User, and checks whether
that user’s result is within 9 ppb from their median qualifying test.
In order to be a Qualied user for User Control tests, a health care professional must rst satisfy the following criteria:
over 18 years of age, non-smoker, no known airway disease or chronic cold, preferably no allergies or asthma. That
user must create a username for themselves, and then perform four User Control tests each separated by at least 12
hours. The rst three tests determine whether this user qualies: they must all be below 40 ppb, and the dierence
between the lowest and highest result must be less than 10 ppb. If these conditions are met, this user is qualied
and their fourth test determines their QC User Status. Its result, and all future results for this user, are compared to
the median result from the rst three tests. If the rst three tests do not meet the qualication criteria, this user is
disqualied and a new user must be created.
Perform a QC Test
1. Touch the QUALITY CONTROL STATUS button from the Settings screen or the title bar at the top of the screen.
2. Touch the SELECT QC USER button.
3. Select the user listed in the SELECT USER FOR TEST window that corresponds to the actual user that will
perform a test. If the user is not listed, create a new user by selecting the NEW USER button and following the
instructions in the Create QC User section of this chapter.
4. Once a user is selected, touch SELECT TEST button.
5. Follow the instructions in Chapter 5: Perform FeNO Measurement to perform a QC test
NOTE: Each day, the system requires a valid User Control and Negative Control test. Any user can perform the
Negative Control breath maneuver, since their breath sample is not being analyzed.
Device QC Status
oPassed - the most recent User Control test and Negative Control test were done within the past 24 hours, and
both passed.
oFailed - the most recent User Control test and Negative Control tests were done within the past 24 hours, but
at least one of those tests failed.
oExpired - No User Control test, or no Negative Control test, has been performed within the past 24 hours.
QC User Status
NOTE: If a QC User result fails, then an alternative QC User should perform the QC Measurements to determine if
the rst QC User may have a changing condition or if the system may require service. If the 2nd QC User fails QC
please contact customer support.
oPassed - latest test is within the expected range for this QC user.
oFailed - latest test is outside the expected range for this QC user.
oExpired - 24 hours or greater have elapsed since the last passing test for this QC user.
oInsucient Data - fewer than four tests have been performed for this QC user.
Create QC User
1. Touch the NEW USER button from the SELECT USER FOR TEST window.
2. Enter a USERNAME. The USERNAME may not contain spaces or match existing users.
3. Touch the SAVE button.

IFU-3393 Rev C Instructions for Use, Fenom Pro
16
R
Chapter 7: Power O Device
It is OK to leave the device powered-on at all times. Your device will automatically go into sleep mode when not being
used. Only power o if you don’t intend to use for extended periods of time.
To power o the device:
1. Hold the POWER button down for at least 1 second.
2. Touch OK on the conrmation window.
NOTE: It is recommended to keep the Fenom Pro device connected to a power supply whenever possible when
powered on.
Chapter 8: Practice Mode
Practice mode is an option for use by a new patient in order to demonstrate the steps for performing a FeNO test.
Results are not recorded in this mode.
IMPORTANT!
Using the Practice mode counts as one exhalation toward the maximum six exhalations
per patient per day and toward the maximum three exhalations per mouthpiece
To access the Practice mode:
1. On the Home screen, select the Start.
2. Touch the Practice button.
3. Attach a new, single-patient-use mouthpiece to the handpiece and review how to use the Fenom Pro device with
the patient. (See Chapter 5: Perform FeNO Measurement for detailed instructions.)
4. The patient inhales then begins exhaling into the mouthpiece for 10 seconds when ready.
5. The patient stops exhaling once the countdown reaches 0 (zero).
6. If training was successful, Good job! displays.
7. If training was unsuccessful, Try again displays.
8. Touch Repeat arrow to perform training again and go back to Step 4, or touch Done if nished to go back to the
Home screen.

IFU-3393 Rev C Instructions for Use, Fenom Pro 17
Chapter 9: Device Setup
Initial Setup
To set up the Fenom Pro device:
1. Remove the device and power cable from the shipping package.
NOTE: Retain all packaging for future transportation of the device.
2. Connect the breath tube to the orange port at the bottom of the handpiece. Ensure the breath tube is fully seated
against the rear surface, as shown in the gure. The second gure shows an improper breath tube connection.
3. Once the breath tube is connected, place the handpiece in the cradle on top of the device.
4. Connect the power cable from the rear panel of the device to an outlet.
(See Table 1 for power connection location.)
The AC Power Indicator displays green when the device is plugged in and powered on.
NOTE: The device should be allowed to charge for at least 4 hours before operating on battery power. The device
can operate normally while charging.
5. Press the Power button to turn on the device.
6. The Device Setup screen will display after powering on. From this screen, set the following device settings:
oSelect Language
oSet Time/Date
oAdd Tests
These settings can be accessed and changed at any time.
7. The Fenom Pro is now ready to begin a test.
Correct Improper

IFU-3393 Rev C Instructions for Use, Fenom Pro
18
R
Language
1. Touch Language on the Settings screen.
2. Select the desired language.
3. A check mark next to the language indicates the
selected language.
Conguration Settings
There are device settings that require conguring based on location and requirements. These settings are accessed
through the Settings icon. (See Table 2: Indicators and Icons.)
Time/Date
1. Touch Time/Date on the Settings screen to set the date
and time on the device.
2. Use the + and - buttons to set the date and time.
3. Touch the AM/PM button to toggle between values.
4. Touch the Time Zone drop-down list and select the
correct time zone.
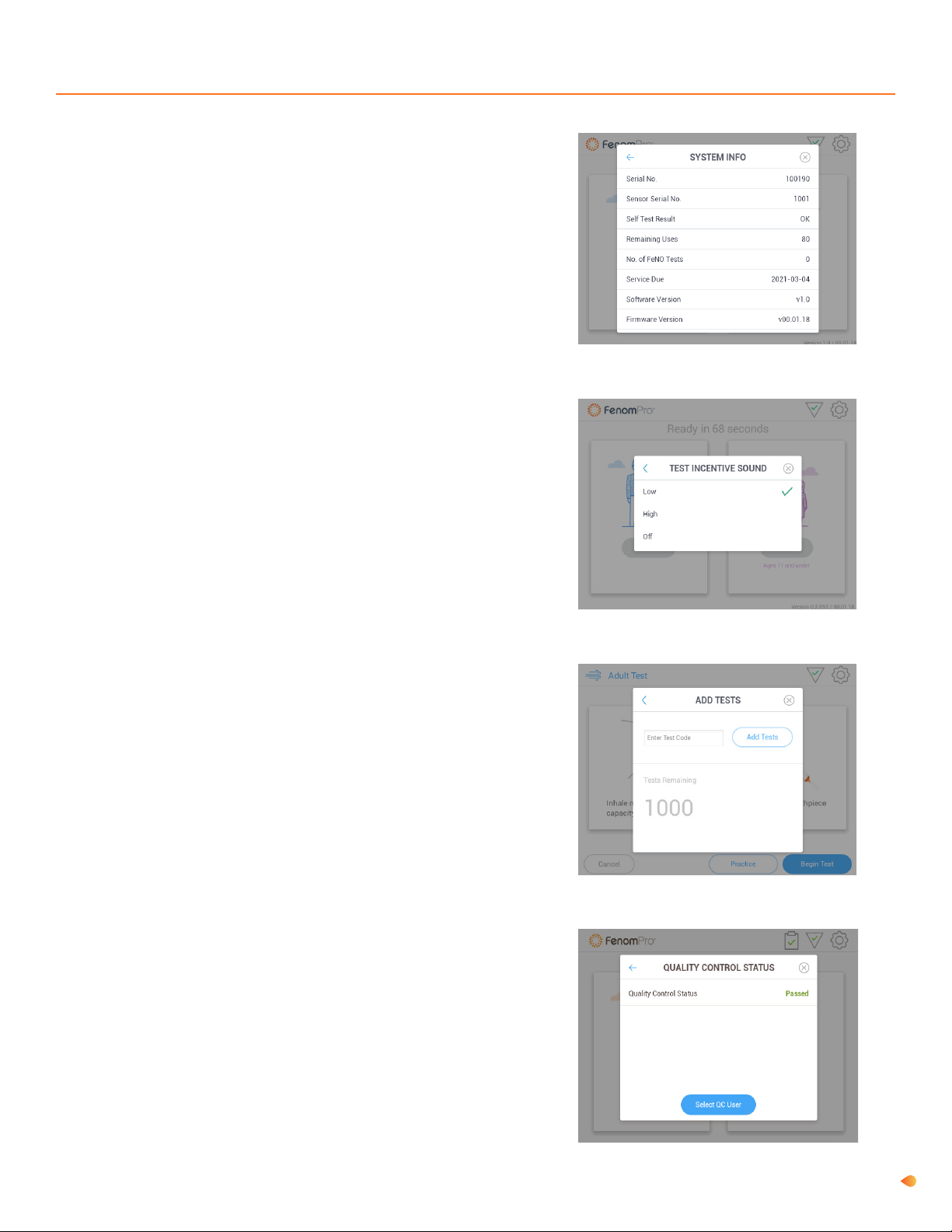
Device Settings
1. The Settings button provides access to set the Time and
Date, select Language, view System Info, select Test
Incentive Sound level, and Add Tests.
IFU-3393 Rev C Instructions for Use, Fenom Pro 19
Add Tests
1. Contact Spirosure support and ask for additional
licensed tests for the Fenom Pro.
2. Enter the code provided by the support representative
and press Add Tests.
Test Incentive Sound
1. Touch Test Incentive Sound on the Settings screen to
set the desired volume level, High, Low, or O.
2. A check mark indicates the current selection.
System Information
1. Touch System Info on the Settings screen to view Device
Serial Number, Licensed Tests (number of licensed tests
remaining), Service Due Date, Software Version, and
Firmware Version.
Quality Control Status
1. Touch Quality Control Status on the Settings screen.
2. See Chapter 6 Quality Control for more instructions..

IFU-3393 Rev C Instructions for Use, Fenom Pro
20
R
Chapter 10: General Care
Follow the recommendations below for cleaning and general care of the Fenom Pro and its accessories.
Operating Conditions
Ensure stable operating conditions by avoiding placement of the device in direct sunlight, near sources radiating heat,
or ventilation. The device operates under the following conditions:
oTemperature range of 15 to 30°C (59 to 86°F)
oAtmospheric pressure range of 106 to 80 kPa
oRelative humidity range of 20 to 80%, non-condensing
Cleaning
oClean the external surfaces of the device with a cloth pre-moistened with 5% bleach solution at the end of each
day of use.
oDO NOT use spray detergents.
Handling
oTake care while handling the device.
oDO NOT drop the device or the handpiece.
oCarry the device by placing ngers in the recessed handle on the back and placing thumb over the top of the
device. Support the device from the bottom with other hand.
Storage
oClean the device before storing.
oStore the device in its original shipping packaging.
oStore the device in a location free from dust, free from excessive moisture or water splash, and away from
excessive heat, cold, or dry conditions.
oDO NOT store the device on tall or unstable surfaces.
oStore mouthpieces in original, unbroken packaging.
IMPORTANT!
Never attempt to open or service the Fenom Pro device or component.
Other manuals for FenomPro
1
Table of contents
Other Spirosure Medical Equipment manuals