Therabody TheraFace Mask User manual

User Manual
Powered by QX-Micro Motor Technology
Clinitally Proven LED Technology

TABLE OF CONTENTS
Language
EN 2- 14
ES 15-26
FR 27-38
IT 39-50
DE 51- 62
ESMX 63-75
FRCA 76-88
SC 89-100
KR 101-112

EN
2
2
Product Overview
TheraFace Mask is the only one of its kind to help relax, restore and rejuvenate your skin. This mask combines 648 full-face Red, Blue, and
Red + Infrared LED lights and has been FDA-Cleared with the Following Indications for Use:
Red Light is intended to treat full-face wrinkles.
Blue Light is intended to treat mild to moderate inflammatory acne.
Red+Infrared Light is intended to treat full-face wrinkles.
The device is safe for use on all skin types (Fitzpatrick Types 1 - 6).
TheraFace Mask
The most advanced LED mask with targeted vibration.
Intended Use
What’s in the Box:
• Red Light is intended to treat full face wrinkles
• Blue Light is intended to treat mild to moderate inflammatory acne
• Red + Infrared Light is intended to treat full face wrinkles
User Manual
Eyeshield (assembled)
Non-chargeable Device Stand
USB-C Cable
Instruction Manual
TheraFace Mask Device
1
1
2
2
33
4
4
5
5

3
EN
3
H
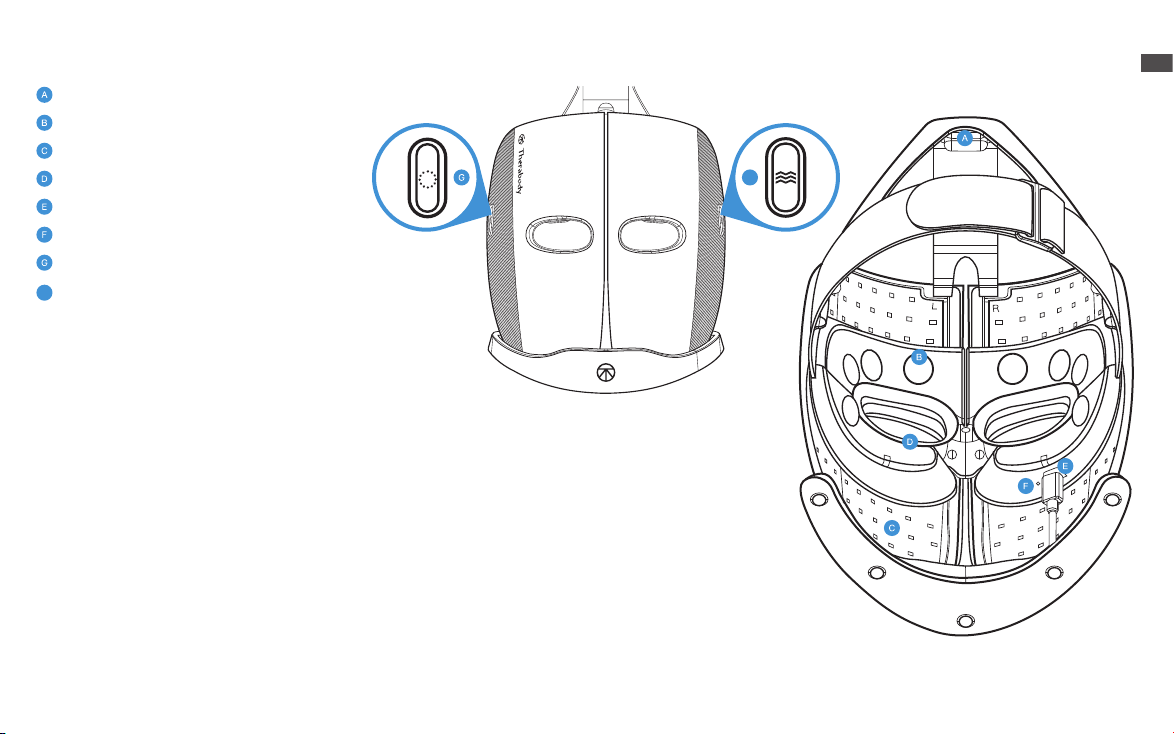
Getting To Know Your TheraFace Mask Device.
Head Massage Units
Face Massage Pads
Multi-color LEDs
Eyeshield
USB-C port
LED indicator
LED button
Vibration button
Getting Started
Long press the LED button (G) to power the device ON and turn on LED light
and vibration massage therapy. The device will guide you through a 9 minute
science-backed treatment that cycles through Red, Red + Infrared, and Blue
LED light therapy in combination with unique vibration patterns. Long press the
same button to power the device OFF.
Long press the vibration button (H) to power the device ON to vibration-only
mode. Short press the vibration button during the treatment to change the
vibration mode. Long press the same button to power the device OFF.
Powering the TheraFace Mask Device On and O.
H

EN
4
4
If the device is not manually powered o by long pressing the LED or vibration
button, the device will automatically shut o after each treatment (LED +
vibration, 9-minute treatment; vibration only, 15-minute treatment).
Automatic Shut O
1. Begin with a clean, dry face
2. Slide the TheraFace Mask eyeshield into the mask through the
protrusions that meet near the nose. Use of the eyeshield is optional.
Using the eyeshield can reduce strain on the eyes from the light.
If you choose not to use the eyeshield, keep your eyes closed
throughout your treatment. Do not look into LED lights during
treatment. *Normal use of TheraFace Mask eyeshields may result in
redness around your eye area. Some redness is normal and should
subside within 5-10 minutes of using TheraFace Mask.
3. Place the device on your face, and adjust both of the Velcro straps to
your desired t
4. To turn on the device, either Long press the LED button (G) to turn
ON LED light + vibration, or the vibration button (H) to turn ON
vibration only
5. Short press the vibration button (H) or LED button to toggle through
each vibration mode (Continuous, Breathing, and Wave)
6. Enjoy your desired treatment.
*Normal use of TheraFace Mask increases blood flow throughout your face, which may result in redness
around your forehead, temples, and bottom of your eyes. Some redness is normal and should subside
within 5-10 minutes of using TheraFace Mask.
Basic getting started steps:
Adjust
Adjust
5
EN
5
Using TheraFace Mask Device
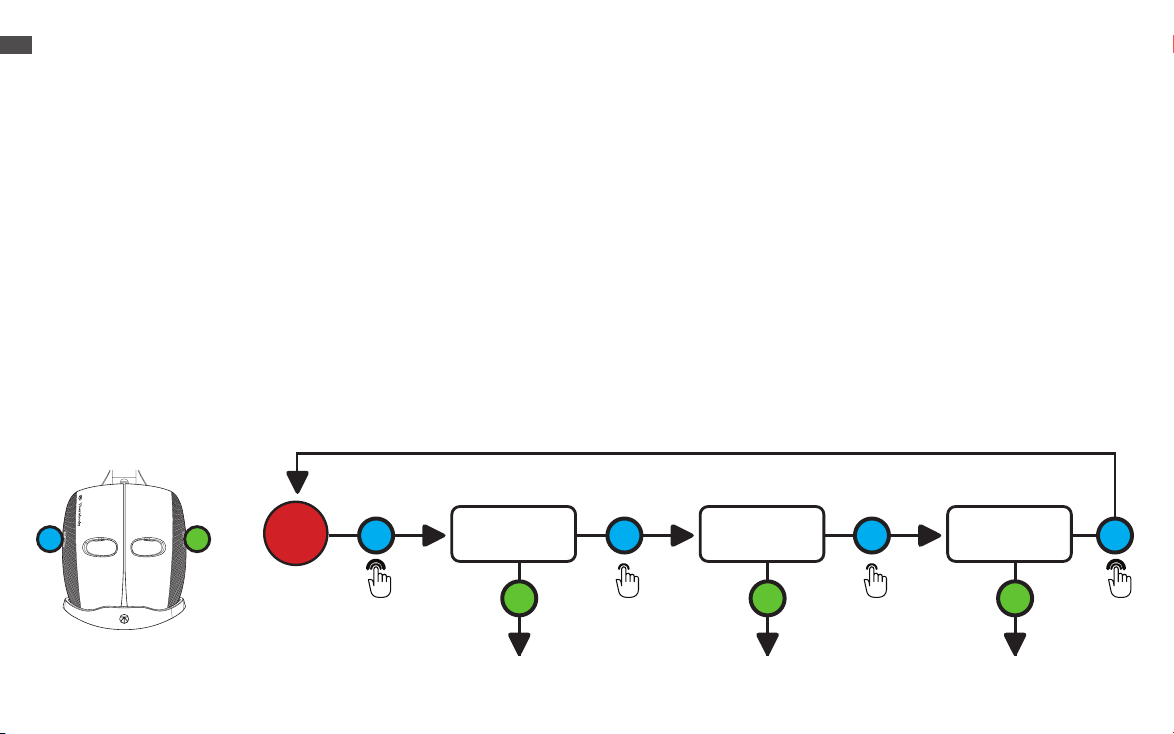
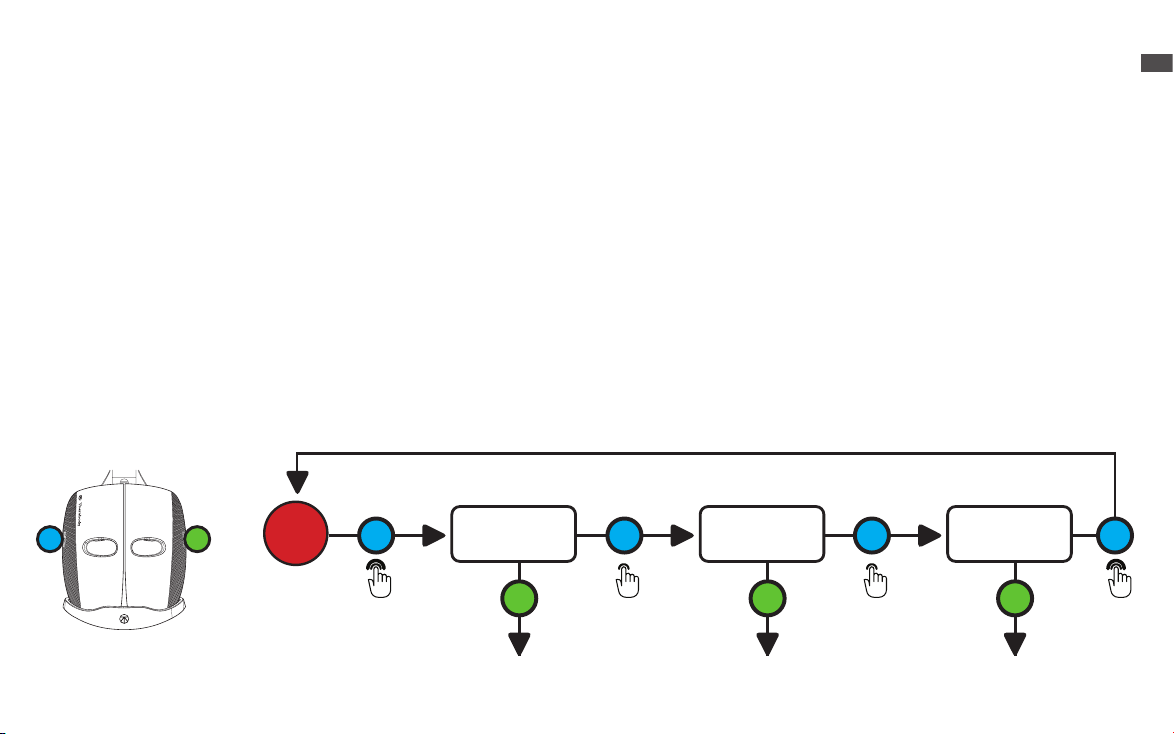
Full Face Light Therapy
1. Place the device securely and comfortably on your face.
2.The TheraFace Mask has three LED light therapy wavelength options: Red, Red + Infrared, Blue.
3.Long press the LED button (G) to turn ON the LED light and vibration. This will turn on the 648 multi-color LED lights around the
face, the eight vibration motors around the eyes, and the nine vibration motors on the top and back of the head. Face and head
vibration therapy accompanies LED light treatment during Red and Red + Infrared treatment. Only head vibration therapy is
available during Blue Light treatment.
4.When you turn ON the device, it will begin an automatic Red Light + vibration treatment. In this mode, the device will guide you
through a science-backed treatment that cycles through Red (3 minutes), Red + Infrared (3 minutes), and Blue (3 minutes) light
therapy in combination with vibration patterns, for a 9-minute treatment.
5.Short press the LED button (G) to toggle through each of the three LED light modes (Red, Red + Infrared, Blue). Each LED light
mode is a 3-minute treatment.
6.Short press the vibration button (H) to toggle through each of the three vibration pattern modes (Continuous, Breathing, and
Wave).
7. Long press the LED button (G) to power the device OFF.
L
ROFF Red Light
Therapy
Blue Light
Therapy
Red + Infrared
Light Therapy
Change
Vibration Mode
Change
Vibration Mode
Change
Vibration Mode
R R RR
L LL
Long LongShort Short
EN
6
6
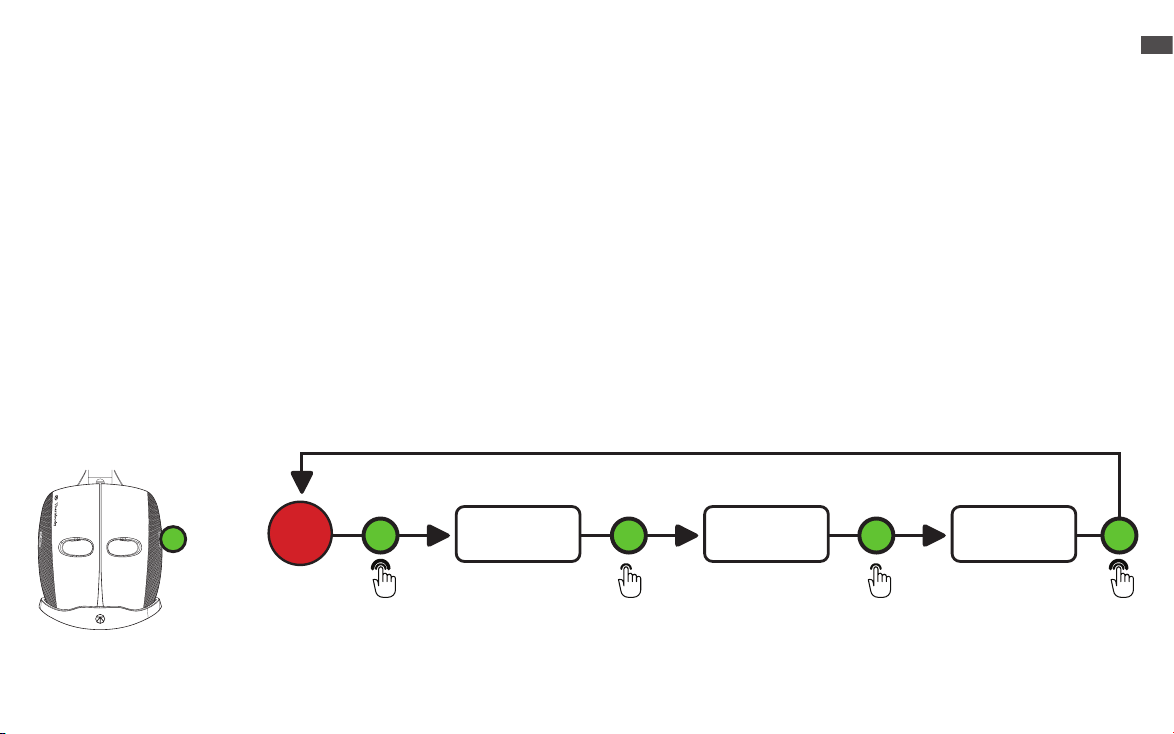
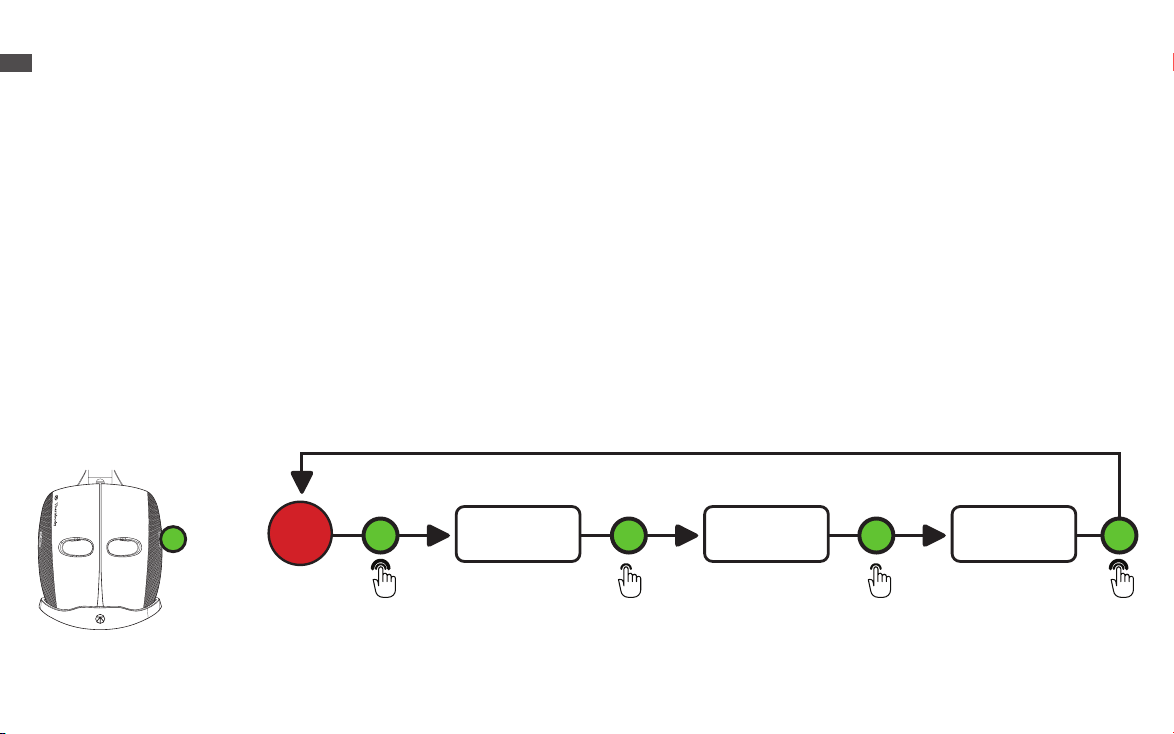
Vibration Therapy
1. Place the device securely and comfortably on your face and long press the vibration button (H) to turn ON vibration only. This will
turn on the eight vibration motors around the eyes and the nine vibration motors on the top and back of the head.
2.When you turn ON the device, it will begin on the Continuous mode. Short press the vibration button (H) to toggle through each
of the three vibration pattern modes (Continuous, Breathing, and Wave). The treatment time is 15 minutes.
3.Short press the vibration button (H) to reach the second vibration mode, Breathing. Allow the motors to guide you through a
breathing exercise. First, breathe in for ve seconds as the vibration intensity increases. The high-speed vibration is your cue to
begin to exhale. Continue to exhale as the vibration gradually slows.
4.Short press the vibration button (H) to reach the third vibration mode, Wave. Sequential vibration patterns will move from face to
head during the Wave treatment.
5.Long press the vibration button (H) to power the device OFF.
LOFF Continuous
Vibration
Wave
Vibration
Breathing
Vibration
L L LL
Long LongShort Short

7
EN
7
Device Maintenance
The following maintenance instructions are important to ensure that
your device continues to work as it was designed. Failure to follow
these instructions may cause your device to stop working.
Care and Cleaning
Charging:
1. Wipe the device clean with a disposable cloth after each use.
2. Before cleaning, power the device OFF by long pressing the LED
light button (G) or vibration button (H); ensure that your device is
not connected to a charging cable or power source before cleaning.
3. Visually inspect the device for any obvious signs of debris build-up.
4. Wipe o any visible dirt with a disposable cloth moistened with a
mild detergent. Be sure to wipe the interior surfaces of the mask.
Next, use a soft cloth stained with 70% isopropyl alcohol to wipe
and disinfect the mask for three (3) minutes, especially the inner
surface of the mask. Repeat this process three times. Finally, wipe
the disinfectant with a clean, dry cloth or towel until there is no
visible residue. Do not submerge the device in water or apply
excessive cleaning solution or disinfectant when cleaning.
5. If you use the TheraFace Mask eyeshield during your treatment,
wipe clean.
6. After cleaning, allow the device to dry thoroughly before storing or
beginning another treatment. A properly cleaned device should
have no visible signs of debris or moisture.
• To check the battery level, short press the LED light
button (G) or the vibration button (H).
• Each light color corresponds with a battery level:
• Orange: Low-charge
• ○ Blue: Mid-charge
• ○ Green: Full-charge
• Before charging the TheraFace Mask, power the
device OFF by long pressing the LED button (G) or
vibration button (H).
• Connect the TheraFace Mask to the USB-C cable.
The port is located inside the mask.
• The battery level is displayed next to the USB-C
port.
• The LED will flash to indicate that the device is
connected properly and charging. This light will turn
green when charging is complete.
TheraFace Mask Device After Care & Cleaning
Note: If using an alternative USB-C cable, ensure that it is from a
trusted source and has not suered any structural damage. Do not
try to use the device while charging.

EN
8
8
Background
TheraFace Mask Product Warnings and Guidance
(Precautions and Contraindications)
Therabody○ products are designed to unlock the body’s natural ability to achieve health and well-being. Through science
and technology, the Therabody portfolio allows people to access the therapeutic benets of dierent natural phenomena to
meet both their needs and preferences. There will be times when it is advisable to modify how devices are used (precautions)
or times when it is not appropriate to use certain devices (contraindications). Read the following safety information for
TheraFace Mask in its entirety prior to use.
Read the full Warnings and Guidance prior to using the TheraFace Mask device.
This device is intended for use by people in good health. This device is contraindicated against and should not be used by or on anyone with a history of
epilepsy, seizures or cardiopathy. The TheraFace Mask device is not recommended for anyone with an electronic implanted device (such as a pacemaker),
cardiac arrhythmia, tumors, or acute episodes of inflammatory diseases. The device is not recommended for those who have arteriosclerosis, thromboses,
or implants in the body region being treated. The device should not be used if you have dark brown or black spots, such as large freckles, birthmarks, moles,
or warts, on the area being treated. The device is not recommended if you have eczema, psoriasis, lesions, open wounds, or active infections other than mild
to moderate inflammatory acne, such as cold sores, in the area being treated. Wait for the infected area to heal before using the device. The device should
not be used if you have abnormal skin conditions caused by diabetes or other systemic or metabolic diseases. If you have a history of herpes outbreaks in
the area of treatment, use of the device is not recommended unless you have consulted with your physician and have received preventive treatment. Please
consult your physician prior to using the device if you are pregnant and/or nursing.
Immediately stop using the device at the rst sign of discomfort.
Adult supervision should be provided for those under the age of 18 using this device. If you have any medical considerations, are taking any medications that
cause light sensitivity, or have had any facial surgery or other surgical procedures, please consult your doctor before using the device.
Important Safety Information
General TheraFace Mask Use

9
EN
9
Safety, Precautions, and Contraindications
Contraindications
• ○Skin rash, open wounds, blisters, local tissue inflammation, infection, bruises, or tumors
• Pregnancy/nursing
• ○Abnormal sensations (e.g., numbness)
• Cancer/tumors
• Epilepsy
• Cardiopathy (heart disease)
• Photo allergy or disorder (e.g., Lupus, porphyria)
• Medications that cause light sensitivity
• Medications for severe inflammatory acne
• Extreme sensitivity to light
• Melasma or hyperpigmentation (especially if exacerbated by mild warmth)
• Suspicious lesions or skin cancer — please consult your physician
• If taking or using any retinol or other sun-sensitive medications, products, or benzoyl
peroxide, do not use infrared light
• Allergy to the device material (Lycra fabric and medical grade transparent TPE and silicone)
The following are circumstances where the potential risks may outweigh the benets. Consult a
medical professional before use.
These recommendations are derived from consultation with medical experts and published
research regarding precautions and contraindications as of the printing date. For up-to-date
information, please visit us online at https://www.therabody.com/us/en-us/precautions-and-
contraindictions.html.
TheraFace Mask LED light therapy (Red LED,
Red+IR LED, and Blue LED Therapies)
Due care is required in these circumstances and device use may need to be modied.
Consult with a medical professional if you currently have or suspect you may have any of
the following conditions or if you have any questions.
• ○ Recent injury, surgery, or facial treatment, including neurotoxin, dermal ller,
microneedling, laser, and chemical peel until the skin has fully healed.
• ○ Current Herpes Simplex Virus breakout
• ○ Broken skin
• ○ Retinol application before use of red LED light
Precautions
Precautions
These recommendations are derived from consultation with medical experts and published research
regarding precautions and contraindications as of the printing date. For up-to-date information, please
visit us online at https://www.therabody.com/us/en-us/precautions-and-contraindictions.html.
TheraFace Mask Vibration Therapy
Due care is required in these circumstances and device use may need to be modied. Consult with a
medical professional if you currently have or suspect you may have any of the following conditions or if
you have any questions.
• ○ Recent injury, surgery, or facial treatment, including neurotoxin, dermal ller, microneedling, laser,
and chemical peel until the skin has fully healed.
• ○ Current Herpes Simplex Virus breakout
• ○ Broken skin
• ○ Hypertension (controlled)
• ○ Abnormal sensations (e.g., numbness)
• ○ Sensitivity to pressure
• ○ Medications that may alter sensations
Contraindications
• ○ Skin rash, open wounds, blisters, local tissue inflammation, infection, bruises, or tumors
• ○ Active inflammatory acne breakout
• ○ Bone fracture or myositis ossicans
• ○ Hypertension (uncontrolled)
• ○ Acute or severe cardiac, liver, or kidney disease
• ○ Neurologic conditions resulting in loss or altered sensation
• ○ Bleeding disorders
• ○ Recent surgery or injury
• ○ Connective tissue disorders
• ○ Peripheral vascular insuciency or disease
• ○ Medications that thin the blood or alter sensations
• ○ Direct placement over surgical site or hardware
• ○ Extreme discomfort or pain
• ○ Pacemaker, ICD, or history of embolism
The following are circumstances where the potential risks may outweigh the benets.
Consult a medical professional before use.
Limited Warranty
For full warranty information, please visit www.therabody.com/warranty. To request a copy of the warranty by mail, you may send a request to the following address:
Therabody - Warranty
Attn: Customer Service
6100 Wilshire Blvd. Ste 200
Los Angeles, Ca. 90048

EN
10
10
Please note, this is not a return address or a retail location. No Therabody products or
packages will be accepted at this location.
Customers who are in need of product support should visit https://www.therabody.com/us/
en-us/support/support.html for the available contact methods.
FDA-Cleared
Limited Warranty Only With Authorized Retailer Purchase
©2023 Therabody, Inc. All Rights Reserved.
Patents and Pending Patents at www.therabody.com/patents.
Distributed by:
Therabody International Limited, Marine House, Clanwilliam Place, Dublin, Ireland
Therabody UK Limited, 55 Ludgate Hill, 2nd Floor, London EC4M7JW
Therabody Australia Pty. Ltd., The Commons South Yarra, 11 Wilson Street, South Yarra VIC 3141
1. USE ONLY AS INSTRUCTED. Use the TheraFace Mask as described in the TheraFace Mask User Manual only. Use only Therabody recommended accessories and replacement parts. Do not carry out any
maintenance other than as advised by Therabody.
2. NOT FOR CHILDREN. The TheraFace Mask and charger are not intended for use by young children or persons with reduced physical, sensory, or reasoning capabilities, or lack of experience and knowledge. The
TheraFace Mask is not to be used as a toy. Do not play with, bend, or pull the electrical components. Advise children not to play with the TheraFace Mask or charger.
3. CHARGING LOCATIONS. Charge the TheraFace Mask with a USB-C charger. The TheraFace Mask should be charged indoors in a well-ventilated, dry location. Do not charge the TheraFace Mask outdoors, in a
bathroom, or within 10 feet (3.1 meters) of a bathtub, shower, or pool. Do not use the TheraFace Mask or charger on wet surfaces, and do not expose the charger to moisture, rain, or snow. Do not use the TheraFace Mask
or its compatible charger in the presence of explosive atmospheres (gaseous fumes, dust, or flammable materials). Sparks may be generated, possibly causing a re.
4. DO NOT OVERCHARGE. Do not leaave the device connected to the charger for more than one hour after the battery has been fully charged. The battery includes a protection system to avoid the risk of
overcharging. However, overcharging may reduce its life over time.
5. DO NOT CRUSH, DROP, OR DAMAGE THE DEVICE OR CHARGER. Do not use a charger that has received a sharp blow, been dropped, run over, or damaged in any way.
6. BATTERY CHEMICALS CAUSE SERIOUS BURNS. Never allow the internal battery to come into contact with the skin, eyes, or mouth. If a damaged battery leaks chemicals, use rubber or neoprene gloves to dispose
of it. If skin is exposed to battery fluids, wash with soap and water and rinse with vinegar. If eyes are exposed to battery chemicals, immediately flush with water for 20 minutes and seek medical attention. Remove and
dispose of contaminated clothing.
7. DO NOT SHORT CIRCUIT. A battery will short circuit if a metal object makes a connection between the positive and negative contacts on the battery or the 16V connector. Do not place a battery near anything that
may cause a short circuit, such as coins, keys, or nails in your pocket. A short circuited battery may cause re and personal injury.
8. DEVICE DISPOSAL. This device contains a lithium-ion battery, and care must be taken upon disposal of the device. Before disposal of this device, please review your local laws and requirements surrounding Lithium
Ion Battery disposal. The preferred method of disposal is recycling the whole device.
9. SERVICE. If the device is not working properly, has received a sharp blow, or has been dropped, damaged, left outdoors, or dropped into water, then do not use it. Do not attempt to repair or disassemble the device
which may result in an electric shock or re.
10. USAGE. Discontinue use of the TheraFace Mask if you are feeling severe pain. If at any point during the treatment you feel pain or discomfort beyond what’s expected from an ice pack or a hot shower, stop the
treatment immediately and remove the device. Discontinue use of the device if it overheats or becomes uncomfortably hot.
11. THERABODY USB-C CABLE CARE. Unplug the cable when not in use. Pull the plug, not the cable, to reduce the risk of damage to the electrical plug and cable. Store cable to ensure it is not stepped on, tripped
over, or otherwise subjected to damage or stress. Keep the cable away from heated surfaces, oil, and sharp edges. Never operate this appliance if it has a damaged cable or plug if it is not working properly, if it has been
dropped or damaged, or dropped into water. Do not stretch the charger cable or place the cable under strain. Do not handle the cable with wet hands. For long-term storage, store with a fully charged battery. Therabody
is not responsible for damages that may occur due to the use of third-party chargers.
12. DO NOT OPERATE UNDER BLANKET AND PILLOW. Excessive heating can occur and cause re, electric shock, or injury.
13. BATTERY. There is only one correct USB-C insertion position on the device. Do not force the cable into place. The Therabody logo should be facing upright when inserted.
14. STORING THE TheraFace Mask, BATTERY, AND CHARGING CABLE. Store in a cool, dry place. Only charge the TheraFace Mask when the ambient temperature is between 0°C/32°F and 40°C/104°F. Do not store
the TheraFace Mask, batteries, or charger where temperatures may exceed 40°C/104°F, such as in direct sunlight, in a vehicle, or in a metal building during the summer.
15. DEVICE CARE. The TheraFace Mask is NOT waterproof. The device is not machine washable. Do not place or store the device where it can fall or be pulled into a tub or sink. Do not place in or drop into water or other
liquid. Do not reach for an appliance that has fallen into or come into contact with water. Unplug immediately. Clean the device according to the instructions found in the “After Care and Cleaning” section above.
16. DO NOT DISASSEMBLE. Disassembly or incorrect reassembly may result in the risk of electric shock, re, or exposure to battery chemicals. The warranty will be void if the device, batteries, or charger are
disassembled or if any parts have been removed.
BEFORE USING OR CHARGING THE TheraFace Mask, READ ALL INSTRUCTIONS AND CAUTIONARY MARKINGS IN THIS MANUAL, ON THE CHARGER, AND THE TheraFace Mask. If your
device doesn’t turn on or the battery indicator displays a low battery level, please charge before rst use. The TheraFace Mask is intended for over-the-counter use.
This TheraFace Mask is not intended to diagnose, cure, or prevent diseases. Therabody strives to make the TheraFace Mask as safe for intended use as possible. This is an advanced mechanical tool
with electric components. If the TheraFace Mask and its accessories are not used or maintained properly, there is a risk of re, electric shock, or injury. When using the TheraFace Mask, the following
basic precautions should always be adhered to:
UNIT WARNINGS

11
EN
11
Labels
SYMBOLS DESCRIPTION LOCATION
IP 22 Degree of protection against ingress of water On rating label
Read instructions before use On rating label
Level of protection type BF applied part On rating label
In accordance with Directive 2014/30/ EU
electromagnetic compatibility
On rating label (only in the EU)
In accordance with UK Regulations On rating label (only in the EU)
Therabody, Inc.
Los Angeles, CA 90048
On rating label
Labels
SYMBOLS DESCRIPTION LOCATION
UDI :
(01)
00810036056908
(10)
(21)
2143
00001
Unique Device Identication (UDI) On rating label
Separate collection for waste electrical and
electronic equipment.
Note: For more information about disposal of
equipment, its parts and accessories, please
contact your local distributor.
On rating label
RCM Mark On rating label
Temperature, Operating: 0 ~ 35 °C
Relative Humidity, Operating: ≤85 %
Temperature, Storage: -10 ~ 50 °C
Relative Humidity, Storage/Shipping: ≤85 %
Atmospheric Pressure: 70.0 kPa 106.0 kPa
Operating /Storage environment:
Troubleshooting
ISSUE POSSIBLE CAUSE CORRECTIVE ACTION
Device does not power on 1. Battery level is low Charge the device
2. It still can’t be turned on Contact Therabody
Vibration motor does not turn 1. Motor or connection has been damaged Try charging the device fully, removing the charging cable and
then pressing left button for 3seconds. If problem persists,
contact Therabody
The battery won’t charge 1. The ambient temperature is too high or too low Charge the device when the ambient temperature is between
5° C and 35° C
2. Possible defective battery Contact Therabody
3. Defective charging cable Try using a dierent usb-c cable and adapter

EN
12
12
The TheraFace Mask is intended for use in the electromagnetic environment specied below. The customer or the user of the TheraFace Mask should assure that it is used in such an environment.
IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL ELECTROMAGNETIC ENVIRONMENT GUIDANCE
Electrostatic discharge (ESD)
IEC 61000-4-2 ± 8 kV contact
± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air ± 8 kV contact
± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air Floors should be wood, concrete or ceramic tile. If floors are
covered with synthetic material, the relative humidity should be
at least 30 %.
Electrostatic transient / burst
IEC 61000-4-4 ±2kV for power supply lines
±1kV for input/output lines ±2kV for power supply lines Mains power quality should be that of a typical commercial or
home environment.
Surge
IEC 61000-4-5 ±1kV dierential mode
±2kV common mode ±1kV dierential mode Mains power quality should be that of a typical commercial or
home environment.
Voltage dips, short interruptions and voltage variations on power
supply input lines
IEC 61000-4-11
0% UT; 0,5 cycle g) At 0°, 45°, 90°, 135°, 180°, 225°, 270°and 315°
0 % UT; 1 cycle and 70 % UT; 25/30 cycles Single phase: at 0°
0 % UT; 250/300 cycle
45°, 90°, 135°, 180°, 225°, 270° and 315°
0 % UT; 1 cycle and 70 % UT; 25/30 cycles Single phase: at 0°
0 % UT; 250/300 cycle
Mains power quality should be that of a typical commercial or home
environment. If the user of the TheraFace Mask requires continued
operation during power mains interruptions, it is recommended that
the TheraFace Mask be powered from an uninterruptible power
supply or a battery.
Power frequency (50/60 Hz magnetic eld
IEC 61000-4-8
30 A/m 30 A/m Power frequency magnetic elds should be at levels
characteristic of a typical location in a typical commercial or home
environment.
NOTE UTis the a.c. mains voltage prior to application of the test level.
GUIDANCE AND MANUFACTURER´S DECLARATION ELECTROMAGNETIC EMISSION
The TheraFace Mask is intended for use in the electromagnetic environment specied below. The customer or the user of TheraFace Mask should assure that it is used in such an environment.
EMISSIONS TEST COMPLIANCE ELECTROMAGNETIC ENVIRONMENT GUIDANCE
RF emissions
CISPR 11 Group 1 The TheraFace Mask uses RF energy only for its internal function. There for, its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11 Class B The TheraFace Mask suitable for use in all establishments, including domestic establishments and those directly connected to the public low-
voltage power supply network that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2 Class A
Voltage function / flicker emissions
IEC 61000-3-3 Complies
GUIDANCE AND MANUFACTURER´S DECLARATION ELECTROMAGNETIC IMMUNITY
Guidance and manufacturer´s declaration – electromagnetic emission – for all EQUIPMENT AND SYSTEMS

13
EN
13
The TheraFace Mask is intended for use in the electromagnetic environment specied below. The customer or the user of the TheraFace Mask should assure that it is used in such an environment.
IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL ELECTROMAGNETIC ENVIRONMENT GUIDANCE
Conducted RF
IEC 61000-4-6 3 Vrms
150kHz to 80MHz
6 V in ISM and amateur radio
bands between 0,15 MHz and
80 MHz
3 Vrms
150kHz to 80MHz
6 V in ISM and amateur radio
bands between 0,15 MHz and
80 MHz
Portable and mobile RF communications equipment should be used no closer to any part of the TheraFace Mask, including
cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance:
80 MHz to 800 MHz 800 MHz to 2.7 GHz
where p is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and
d is the recommended separation distance in metres (m).b
Field strengths from xed RF transmitters, as determined by an electromagnetic site survey,ashould be less than the compliance level in each frequency range.b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic is aected by absorption and reflection from structures, objects and people.
Radiated RF
IEC 61000-4-3 10 V/m
80MHz to 2.5GHz
385MHz-5785MHz Test
specications for ENCLOSURE
PORT IMMUNITY to RF
wireless communication
equipment (Refer to table 9 of
IEC 60601-1-2:2014)
10 V/m
80MHz to 2.5GHz
385MHz-5785MHz Test
specications for ENCLOSURE
PORT IMMUNITY to RF
wireless communication
equipment (Refer to table 9 of
IEC 60601-1-2:2014)
a The ISM (industrial, scientic and medical) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13,567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz. The amateur radio bands between 0,15 MHz and 80 MHz are 1,8 MHz to
2,0 MHz, 3,5 MHz to 4,0 MHz, 5,3 MHz to 5,4 MHz, 7 MHz to 7,3 MHz, 10,1 MHz to 10,15 MHz, 14 MHz to 14,2 MHz, 18,07 MHz to 18,17 MHz, 21,0 MHz to 21,4 MHz, 24,89 MHz to 24,99 MHz, 28,0 MHz to 29,7 MHz and 50,0 MHz to 54,0 MHz.
b Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld strength in the location in which the TheraFace Mask is used exceeds the applicable RF compliance level above, the TheraFace Mask should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re¬orienting or relocating the TheraFace Mask.
c Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3V/m.
Recommended separation distances between portable and mobile RF communications equipment and the EQUIPMENT or SYSTEM - for EQUIPMENT and SYSTEMS
Recommended separation distances between portable and mobile RF communications equipment and the TheraFace Mask
The TheraFace Mask is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the
TheraFace Mask can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and the TheraFace Mask as recommended below, according to the maximum output power of the communications equipment
Rated maximum
output of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
outside ISM and
amateur radio bands
d=[
!.#
$!
]
150 kHz to 80 MHz
in ISM and
amateur
radio bands
d=[
!"
#!
]
80 MHz to 800 MHz
d=[
!"
#!
]
80 MHz to 800 MHz
d=[
!
"!
]
0.01 0.12 0.20 0.035 0.07
0.1 0.38 0.63 0.11 0.22
1 1.2 2.00 0.35 0.70
10 3.8 6.32 1.10 2.21
100 12 20.00 35 70
For transmitters rated at a maximum output power not listed above the recommended separation
distance d in metres (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is aected
by absorption and reflection from structures, objects and people.
d=[
!.#
$!
]
d=[
!"
#!
]
d=[
!.#
$!
]
d=[
!
"!
]

EN
14
14
Reporting adverse events to FDA
Use one of the methods below to submit voluntary adverse event reports to the FDA:
• ○Report Online at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home
• ○Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. For help lling out the form, see MedWatchLearn. The form is available at
www.fda.gov/downloads/aboutFDA/reportsmanualsforms/forms/ucm349464.pdf
• ○Call FDA at 1-800-FDA-1088 to report by telephone.
• ○Reporting Form FDA 3500 commonly used by health professionals. The form is available at
www.fda.gov/downloads/aboutFDA/reportmanualsforms/forms/ucm163919.pdf
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device must accept any interference
received, including interference that may cause undesired operation.
MedWatch is the Food and Drug Administration’s (FDA) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical
products, including drugs, biologic products, medical devices, dietary supplements, infant formula, and cosmetics.
If you think you or someone in your family has experienced a serious reaction to a medical product, you are encouraged to take the reporting form to your doctor. Your health care provider can provide clinical
information based on your medical record that can help FDA evaluate your report.
However, we understand that for a variety of reasons, you may not wish to have the form lled out by your health care provider, or your health care provider may choose not to complete the form. Your health
care provider is not required to report to the FDA. In these situations, you may complete the Online Reporting Form yourself.
You will receive an acknowledgement from FDA when your report is received. Reports are reviewed by FDA sta. You will be personally contacted only if we need additional information.
Submitting Adverse Event Reports to FDA
FCC Caution:

15
ES Descripción general del producto
TheraFace Mask
La máscara LED más avanzada con vibración localizada.
Uso previsto
• La luz roja puede reducir la aparición de arrugas faciales completas
• La luz azul puede reducir la aparición de acné de leve a moderado
• La luz roja + infrarroja puede reducir la aparición de arrugas faciales completas
TheraFace Mask puede proporcionar los siguientes benecios:
El dispositivo es seguro para su uso en todo tipo de pieles (tipos de Fitzpatrick 1-6).
User Manual
1
2
3
4
Contenido de la caja:
Protector ocular (montado)
Soporte de dispositivo no recargable
Cable USB C
Manual de instrucciones
Dispositivo TheraFace Mask
1
2
3
4
5
5
ES
16
H
Introducción al dispositivo TheraFace Mask.
Unidades de masaje para la cabeza
Almohadillas de masaje facial
Luces LED multicolores
Protector ocular
Puerto USB C
Indicador LED
Botón LED
Botón de vibración
Guía de inicio rápido
Mantén pulsado el botón LED (G) para encender el dispositivo y encender la
terapia de masaje con luz LED y vibración. El dispositivo te guiará a través de un
tratamiento avalado cientícamente de 9 minutos que recorre las terapias de
luz LED roja, roja e infrarroja y azul en combinación con patrones de vibración
únicos. Mantén pulsado el mismo botón para apagar el dispositivo.
Mantén pulsado el botón de vibración (H) para encender el dispositivo con
el modo de solo vibración. Pulsa brevemente el botón de vibración durante
el tratamiento para cambiar el modo de vibración. Mantén pulsado el mismo
botón para apagar el dispositivo.
Encendido y apagado del dispositivo TheraFace Mask.
H

17
ES
1. Lávate y sécate bien la cara
2. Desliza el protector ocular TheraFace Mask dentro de la máscara a través de
las protuberancias que se unen cerca de la nariz. El uso del protector ocular es
opcional. El uso del protector ocular puede reducir la tensión en los ojos debido
a la luz. Si decides no usar el protector ocular, mantén los ojos cerrados durante
todo el tratamiento. No mires las luces LED durante el tratamiento. *El uso normal
de los protectores oculares de TheraFace Mask puede provocar enrojecimiento
alrededor del área de los ojos. Es normal que haya un poco de enrojecimiento
y este debería desaparecer dentro de los 5 a 10 minutos posteriores a usar
TheraFace Mask.
3. Coloca el dispositivo en el rostro y ajusta las dos correas de velcro como desees
4. Para encender el dispositivo, mantén pulsado el botón LED (G) para encender
el modo de luz LED + vibración o el botón de vibración (H) para encender solo el
modo de vibración.
5. Pulsa brevemente el botón de vibración (H) o el botón LED para cambiar entre los
modos de vibración (Continuous, Breathing y Wave)
6. Disfruta de tu tratamiento.
*El uso normal de TheraFace Mask aumenta el flujo sanguíneo en todo el rostro, lo que puede provocar rojeces
alrededor de la frente, las sienes y la parte inferior de los ojos. Es normal que haya un poco de enrojecimiento y este
debería desaparecer dentro de los 5 a 10 minutos posteriores a usar TheraFace Mask.
Si el aparato no se apaga manualmente manteniendo pulsado el botón LED o el de
vibración, el aparato se apagará automáticamente después de cada tratamiento (LED +
vibración, tratamiento de 9 minutos, solo vibración, tratamiento de 15 minutos).
Apagado automático
Pasos básicos para empezar:
Ajustar
Ajustar
ES
18
Uso de TheraFace Mask
Terapia facial integral ligera
1. Colócate el dispositivo de forma segura y cómoda en la cara.
2.La máscara TheraFace Mask tiene tres opciones de longitud de onda de terapia de luz LED: roja, roja + infrarroja, azul.
3.Mantén pulsado el botón LED (G) para encender la luz LED y la vibración. Así encenderás las 648 luces LED multicolores
alrededor de la cara, los ocho motores de vibración alrededor de los ojos y los nueve motores de vibración en la parte superior y
posterior de la cabeza. La terapia de vibración en la cara y la cabeza acompaña al tratamiento con luz LED durante los tratamientos
con luz roja y roja + infrarroja. Durante el tratamiento con luz azul, solo está disponible la terapia de vibración en la cabeza.
4.Cuando enciendas el dispositivo, comenzará un tratamiento automático de luz roja y vibración. En este modo, el aparato te guiará
a través de un tratamiento avalado cientícamente que recorre los ciclos de terapia con luz roja (3 minutos), roja + infrarroja (3
minutos) y azul (3 minutos) en combinación con patrones de vibración para ofrecer un tratamiento de 9 minutos en total.
5.Presiona brevemente el botón LED (G) para alternar entre cada uno de los tres modos de luz LED (Roja, Roja + Infrarroja, Azul).
Cada modo de luz LED es un tratamiento de 3 minutos.
6.Pulsa brevemente el botón de vibración (H) para cambiar entre cada uno de los tres modos de vibración (Continuous, Breathing y
Wave).
7. Mantén pulsado el botón LED (G) para apagar el dispositivo.
L
RApagado Terapia de luz
roja
Terapia de luz
azul
Terapia de luz
roja + infrarroja
Cambiar el modo
de vibración
Cambiar el modo
de vibración
Cambiar el modo
de vibración
R R RR
L LL
Mantener pulsado Mantener
pulsado
Pulsar brevemente Pulsar brevemente
19
ES Terapia de vibración
1. Coloca el dispositivo de forma segura y cómoda sobre el rostro y mantén pulsado el botón de vibración (H) para activar solo la
vibración. Así encenderás los ocho motores de vibración alrededor de los ojos y los nueve motores de vibración en la parte superior
y posterior de la cabeza.
2.Cuando enciendas el dispositivo, comenzará en el modo Continuous. Pulsa brevemente el botón de vibración (H) para cambiar
entre cada uno de los tres modos de vibración (Continuous, Breathing y Wave). El tiempo de tratamiento es de 15 minutos.
3.Pulsa brevemente el botón de vibración (H) para llegar al segundo modo de vibración, Breathing. Deja que los motores te guíen a
través de un ejercicio de respiración. Primero, inspira durante cinco segundos a medida que aumenta la intensidad de la vibración.
La vibración de alta velocidad es la señal para comenzar a espirar. Continúa espirando a medida que la vibración se ralentiza
gradualmente.
4.Pulsa brevemente el botón de vibración (H) para llegar al tercer modo de vibración, Wave. Los patrones secuenciales de vibración
se moverán de la cara a la cabeza durante el tratamiento Wave.
5.Mantén pulsado el botón de vibración (H) para apagar el dispositivo.
LApagado Continuous
Vibración
Ondas
Vibración
Respiración
Vibración
L L LL
Mantener pulsado Mantener
pulsado
Pulsar brevemente Pulsar brevemente
Table of contents
Languages: