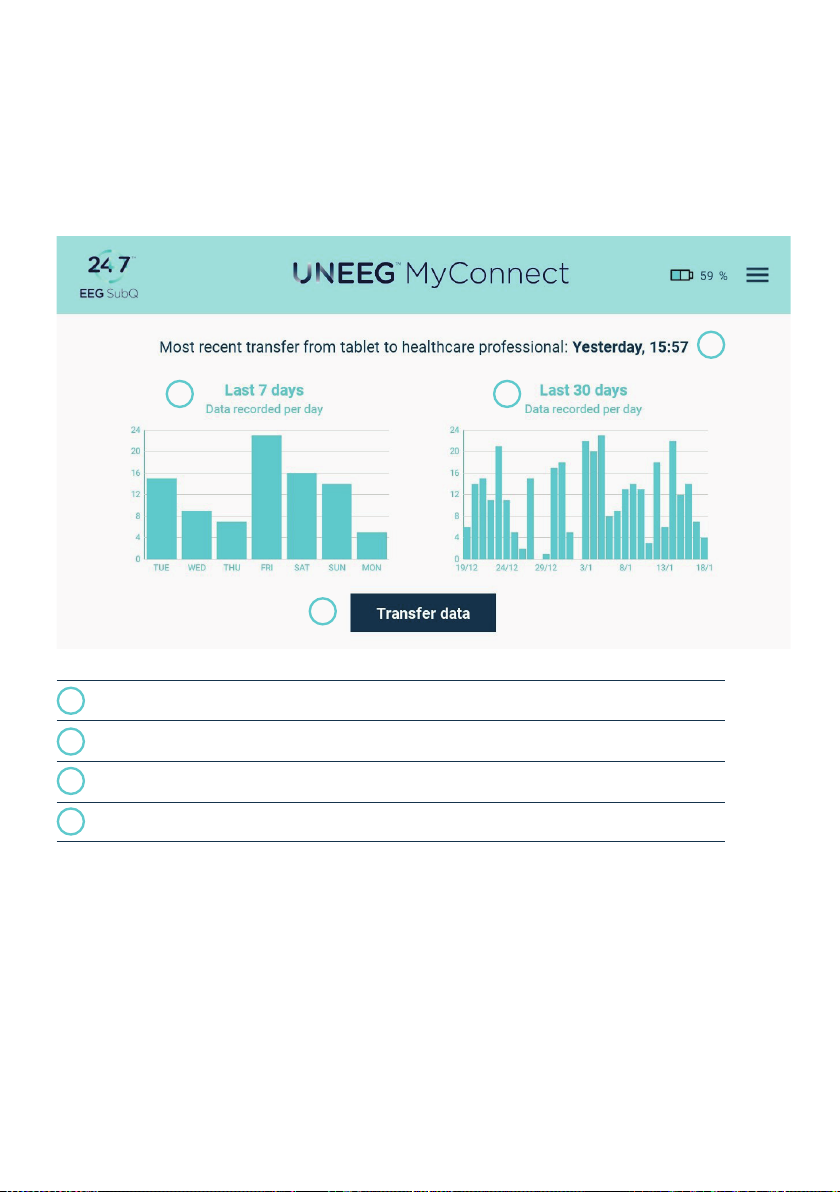
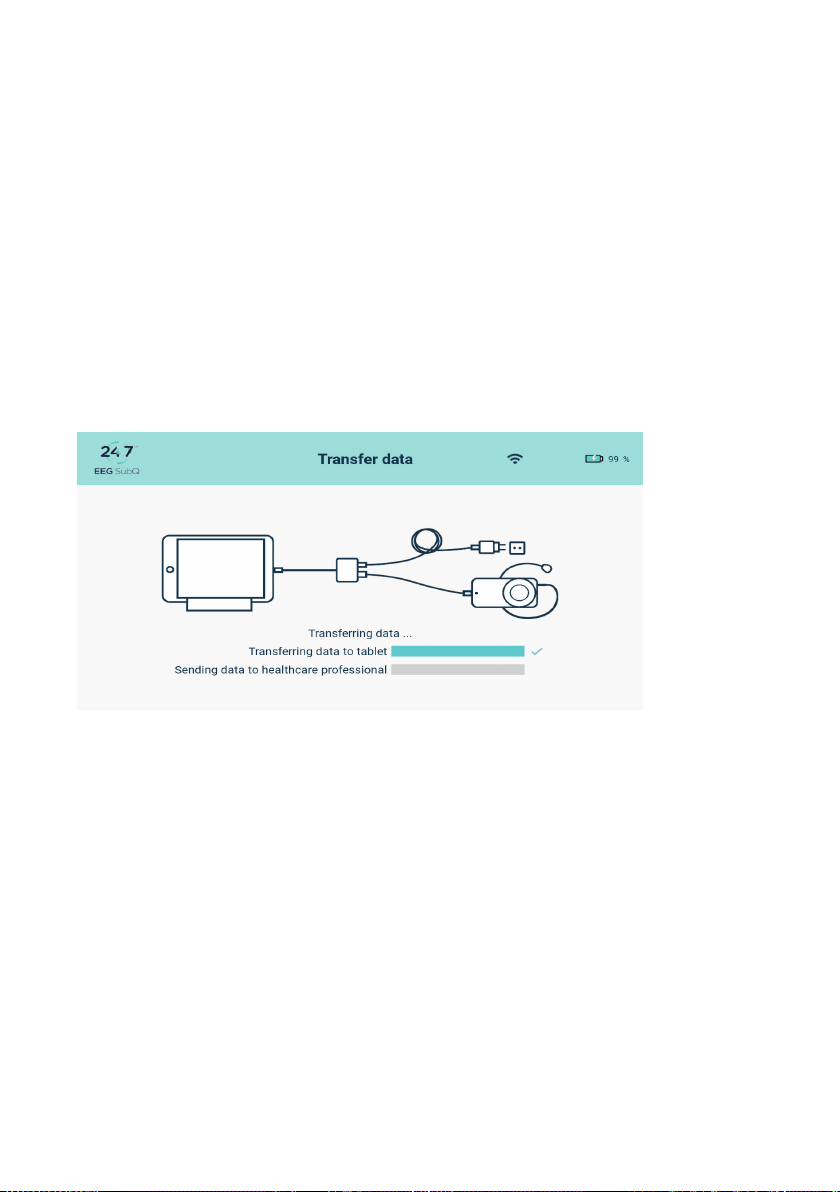
2| USER MANUAL FOR USER | ENGLISH
UNEEG™ MyConnect
TABLE OF CONTENTS
1. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.1 Intended use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.2 Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.3 Intended user and population . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.4 Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.5 Warnings and precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
1.6 Side eects. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2. PRODUCT DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2.1 Requirements for using UNEEG™ MyConnect. . . . . . . . . . . . . . . . . . . . . . . 4
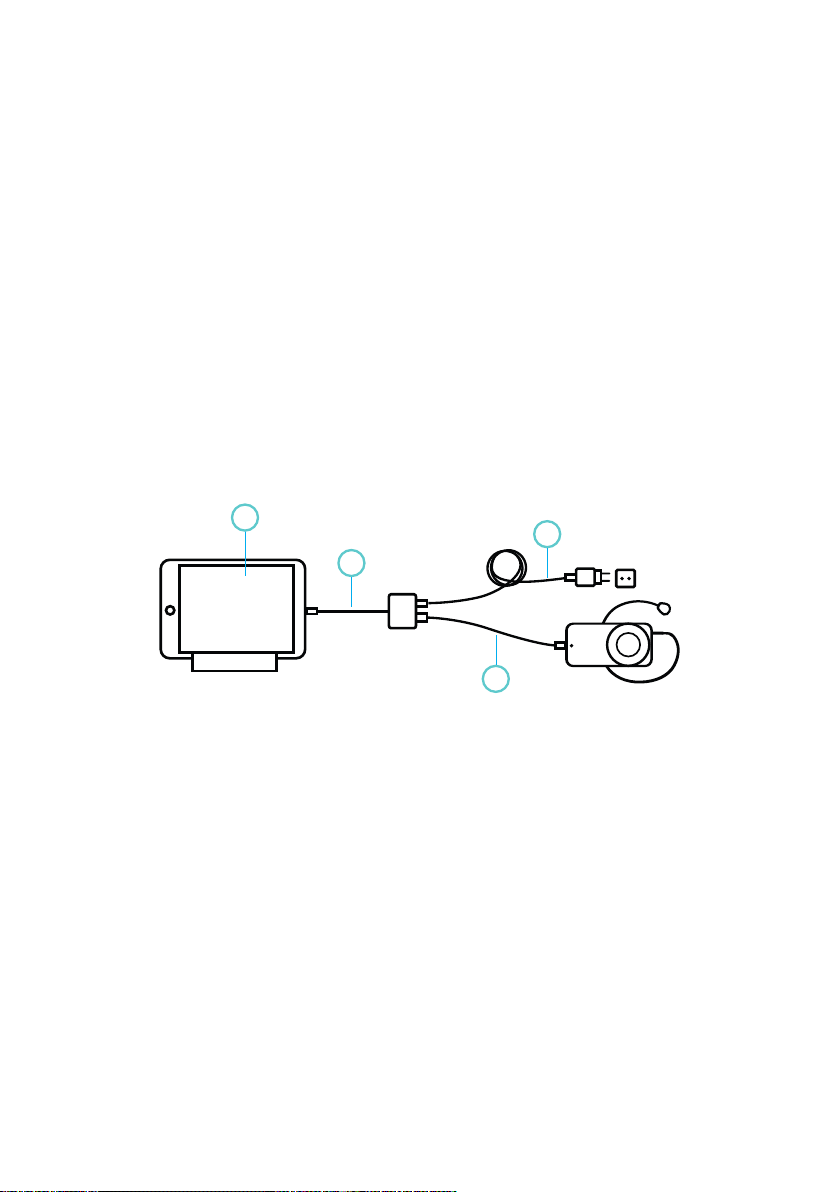
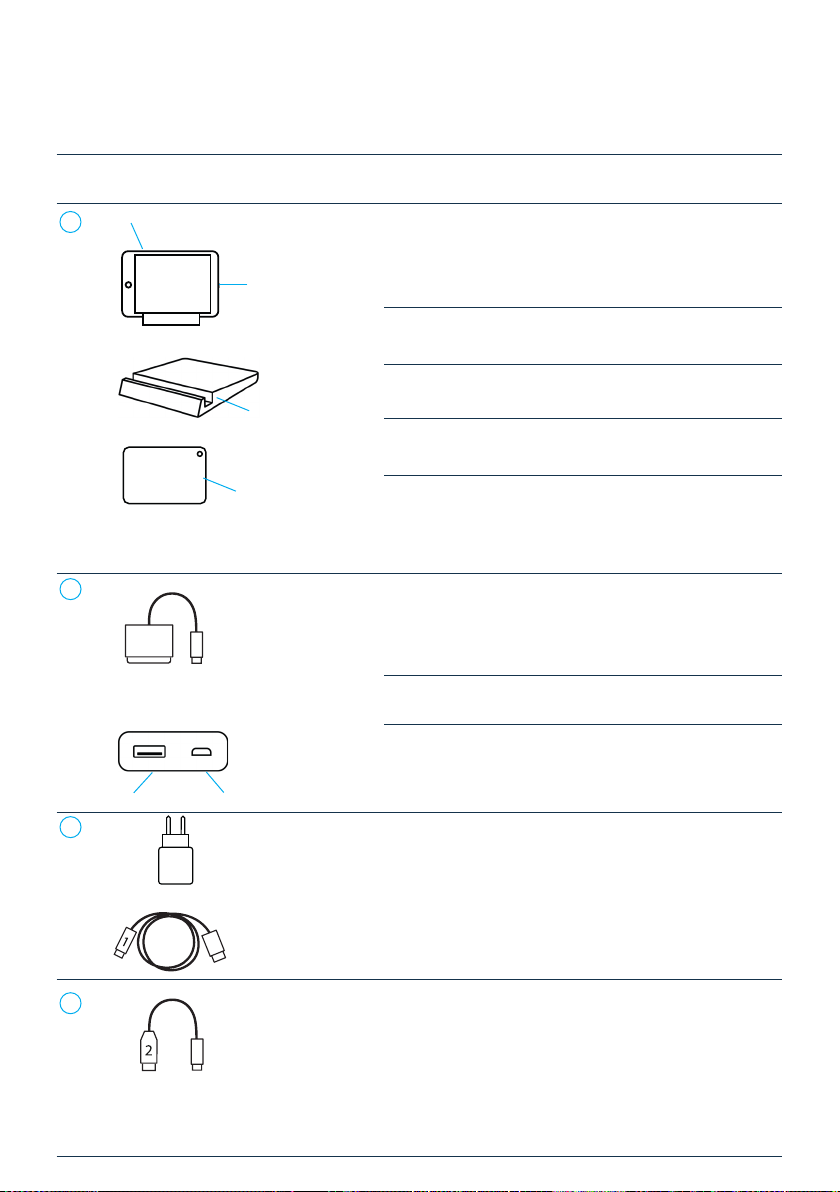
2.2 UNEEG™ MyConnect device description . . . . . . . . . . . . . . . . . . . . . . . . . 4
2.3 Compatible products. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
3. SYMBOLS AND LABELLING. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
4. USING THE SYSTEM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
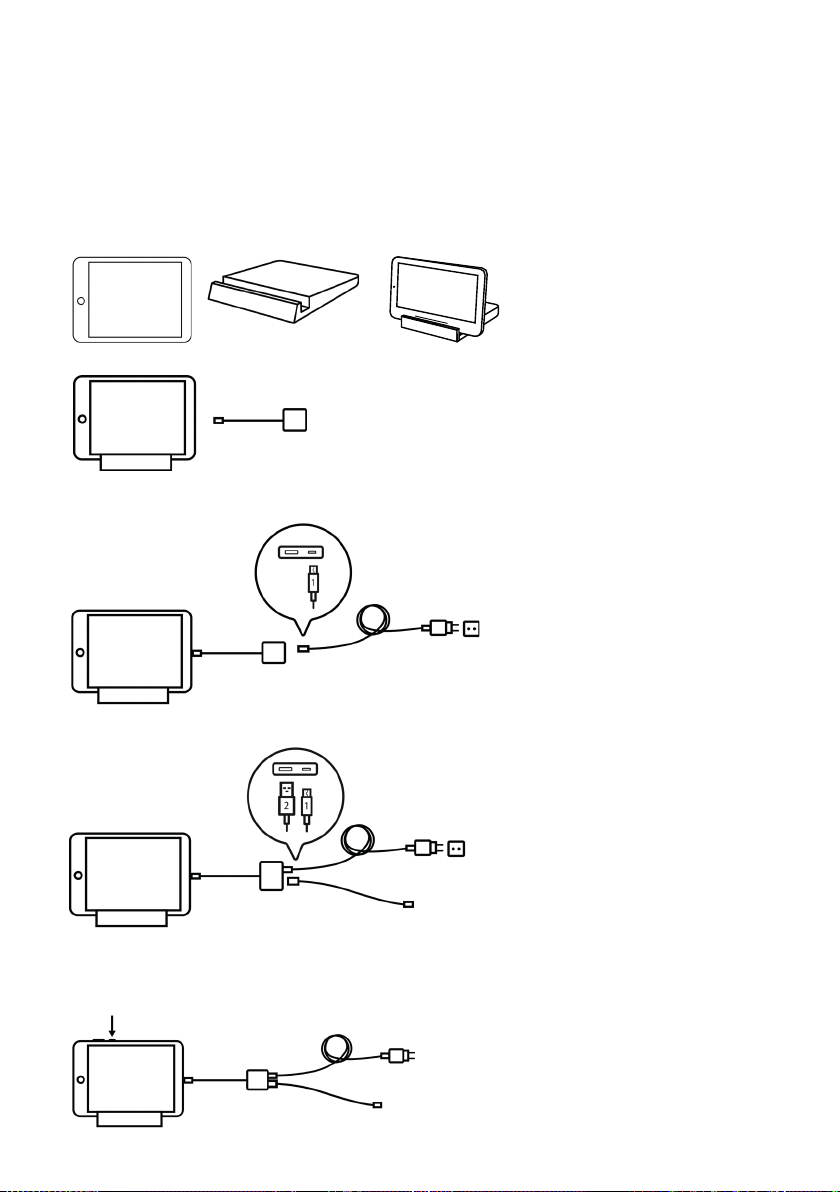
4.1 How to assemble your equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
4.2 Daily use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
4.3 Charging the tablet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
4.4 Serious incident reporting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
5. MAINTENANCE AND DISPOSAL OF EQUIPMENT . . . . . . . . . . . . . . . . . . . . . 11
5.1 Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
5.2 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
5.3 Return of the equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
5.4 Repairs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
6. TROUBLESHOOTING. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
7. TECHNICAL DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
7.1 Environmental conditions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
7.2 Data protection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15