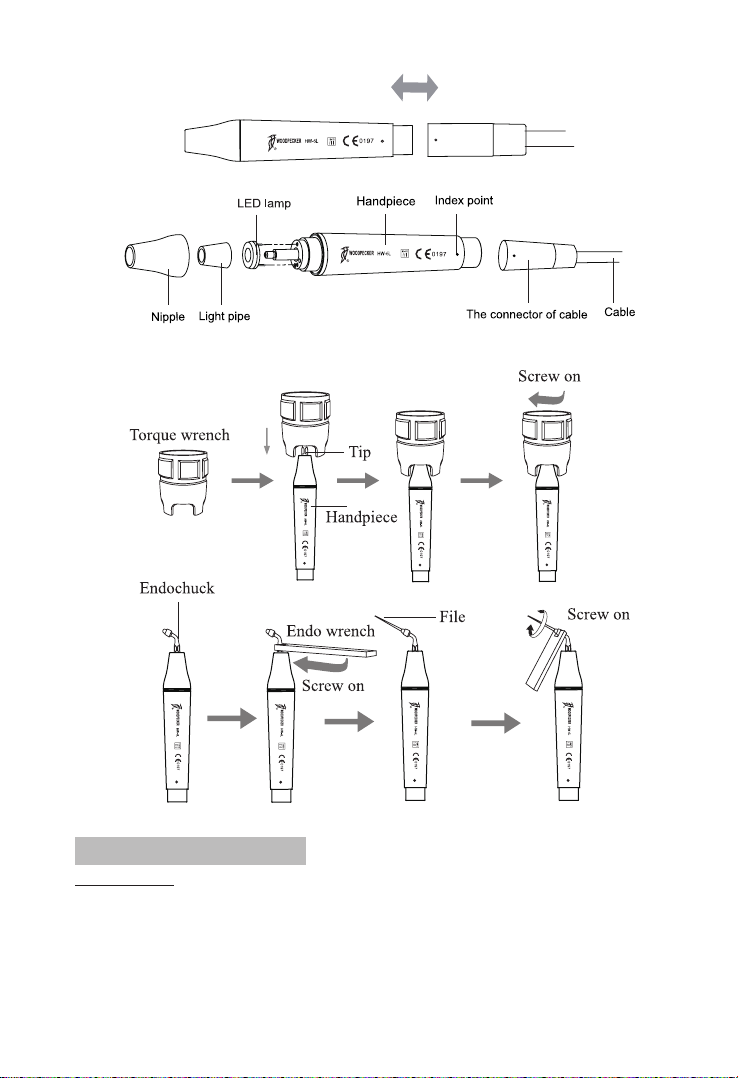
8
4. Cleaning, Disinfection and Sterilization
The cleaning, disinfection and sterilization of handpiece, tip, and
wrench (include torque wrench and Endo wrench) are as follow.
Unless otherwise stated, they will be hereinafter referred to as
“products”.
Warnings
The use of strong detergent and disinfectant (alkaline pH>9 or acid
pH <5) will reduce the life span of products. And in such cases, the
manufacturer takes no responsibility.
Do not clean the handpiece with ultrasound cleaning machine.
This device shall not be exposed to high temperature above 138℃.
Processing limit
The products have been designed for a large number of sterilization
cycles. The materials used in manufacture were selected accordingly.
However with every renewed preparation for use, thermal and chemical
stresses will result in ageing of the products. The maximum number of
sterilizations for handpiece is 600 times. For tips, it is 300 times. And for
wrench, it is 1000 times.
4.1 Initial processing
4.1.1 Processing principles
It is only possible to carry out effective sterilization after the
completion of eective cleaning and disinfection. Please ensure that, as
part of your responsibility for the sterility of products during use, only
sufficiently validated equipment and product-specific procedures are
used for cleaning/disinfection and sterilization, and that the validated
parameters are adhered to during every cycle.
Please also observe the applicable legal requirements in your country
as well as the hygiene regulations of the hospital or clinic,especially with
regard to the additional requirements for the inactivation of prions.
4.1.2 Post-operative treatment
The post-operative treatment must be carried out immediately, no
later than 30 minutes after the completion of the operation. The steps are
as follows:
1. Let the Ultrasonic scaler works for 20-30 seconds under irrigation
mode to ush the handpiece and tip;
2. Remove the handpiece from the Ultrasonic scaler, and rinse away
the dirt on the surface of product with pure water (or distilled water/
deionized water);