zebris Medical GmbH FDM Table of contents Page 1/36
Table of contents
1Introduction..............................................................................................................................................2
1.1 Manufacturer and sales ........................................................................................................................3
1.2 Conventions and symbols used............................................................................................................3
1.4 Intended purpose of the FDM System..................................................................................................4
1.5 Personal safety, warnings and prohibitions ..........................................................................................5
1.6 System components..............................................................................................................................7
1.7 Computer requirements ........................................................................................................................7
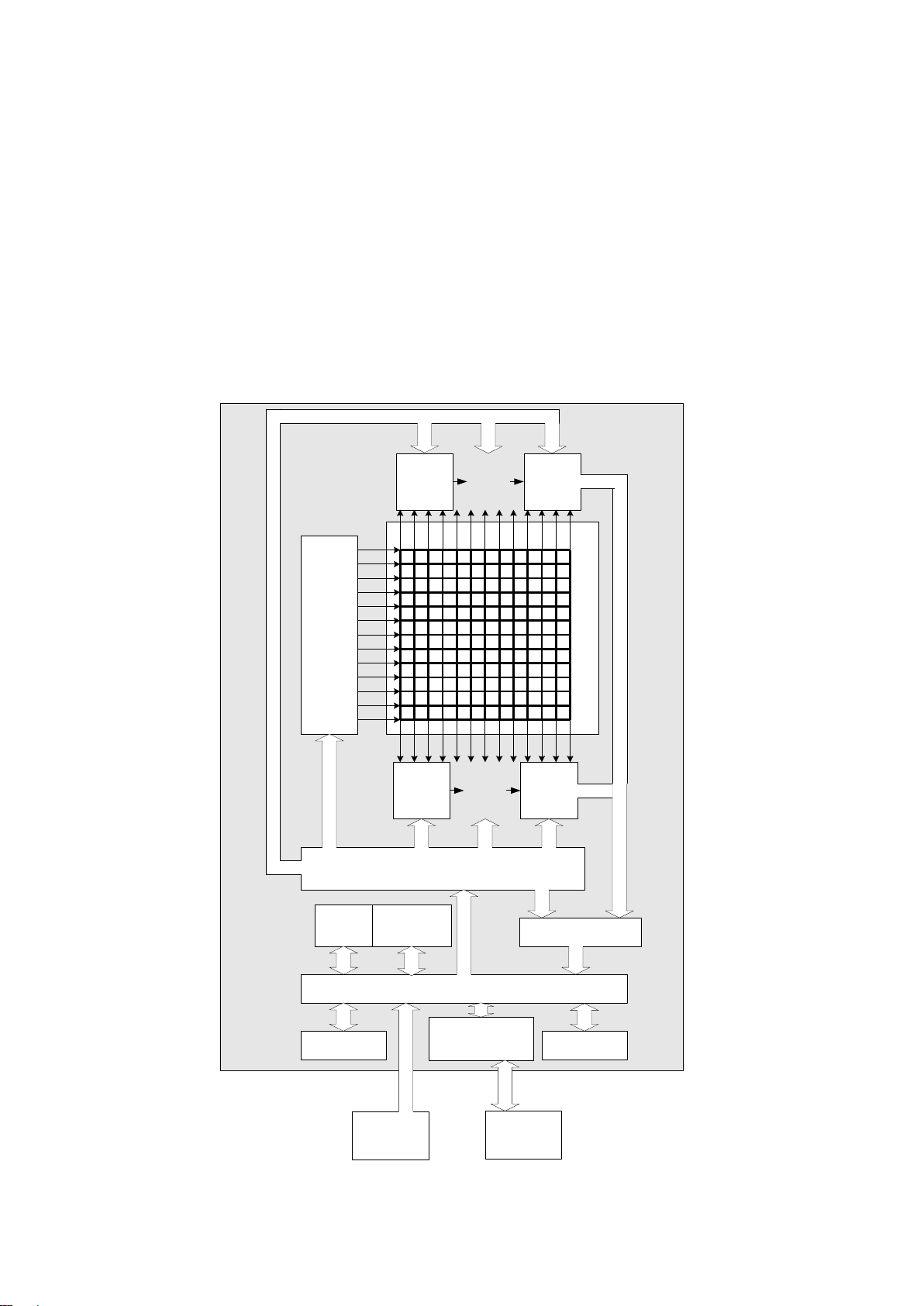
1.8 Measuring principle...............................................................................................................................8
2Technical data..........................................................................................................................................9
2.1 General specification for the FDM-Sensor............................................................................................9
2.2 Technical data of FDM multi function force plates..............................................................................10
2.3 Technical data of FDM system for stance and gait analysis ..............................................................12
2.4 Specifications for the external power supply unit................................................................................15
3Setup and operation of the FDM System............................................................................................16
3.1 General information.............................................................................................................................16
3.2 Status indicator LED ...........................................................................................................................16
3.3 How to switch the platform On/Off......................................................................................................16
3.4 Setup of the FDM platform..................................................................................................................17
3.5 Setting the system out of operation ....................................................................................................18
4Synchronization of the FDM platform .................................................................................................19
4.1 Synchronizing the FDM Platform with video data (Sync Audio) .........................................................19
4.2 Infrared synchronization with zebris DAB-Bluetooth (EMG)...............................................................20
4.3 Combine two FDM platforms of the same type...................................................................................21
4.4 Synchronization of third party devices with the FDM system .............................................................22
5Recommendations for recording data ................................................................................................27
5.1 Walking range.....................................................................................................................................27
5.2 Data recording.....................................................................................................................................27
5.3 Gait velocity.........................................................................................................................................27
5.4 Posture................................................................................................................................................27
5.5 Acrosclerosis.......................................................................................................................................27
6Maintenance...........................................................................................................................................28
6.1 General maintenance information.......................................................................................................28
6.2 Checking the FDM Sensor..................................................................................................................29
6.3 Cleaning and disinfection....................................................................................................................30
7Storage, transport and disposal..........................................................................................................31
7.1 Storage and transport .........................................................................................................................31
7.2 Disposal ..............................................................................................................................................31
8Safety standards and system classification.......................................................................................32
8.1 Classification acc. to Annex IX of Directive 93/42/EEC......................................................................32
8.2 Safety of medical electrical devices....................................................................................................32
8.3 Connecting the FDM-System to other electrical devices....................................................................32
8.4 Electromagnetic compatibility Guideline & Manufacturer Declaration................................................33
8.5 Declaration of conformity medical platforms.......................................................................................36
8.6 Declaration of conformity non medical platforms................................................................................37