English 5
5. INTRODUCTION
This compressor nebulizer is designed to deliver a prescribed medication
solution to treat patient respiratory disorders, such as asthma, allergies and
bronchitis. The device converts the medication solution into an aerosolized
mist which is inhaled by the patient through the mouthpiece or nosepiece or
mask. Read this manual thoroughly before using the device and store it for
future reference.
6. SPECIFICATIONS
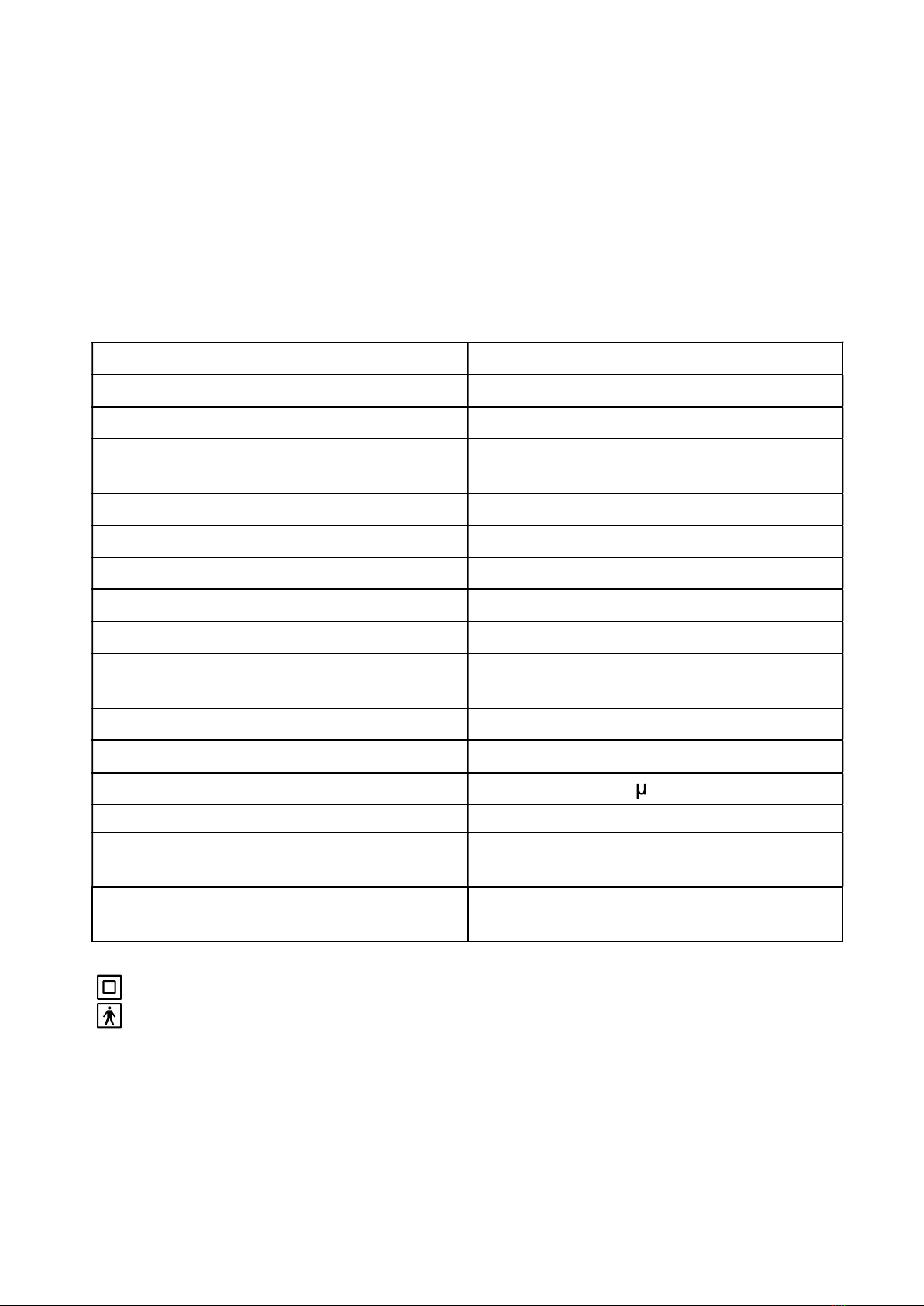
Electrical Requirements 230 V / 50 Hz
Power Consumption Below 60 W
Rated Current Below 0.70 A
Dimensions Length 250mm × Width 118mm ×
Height 175mm
Weight 1.4kg(3.09lbs)
Sound Pressure Level 55 dBA
Max. Compression Pressure 35 psi to 50 psi (241 kPa to 345 kPa)
Neb. Operating Pressure 9-16 psi (62 kPa to 110 kPa)
Liter Flow Range 5-8 L/min
Average Nebulization Rate: Approx. 0.33 ml/min (6 ml medicine
can be nebulized within 18 minutes)
Maximum nebulizer solution capacity 6 ml
Maximum residual medicine volume 0.5 ml
Particle size (MMAD) Approximately 3 m
Operating Temperature/Humidity +10°C to + 40°C, 30% RH to 85% RH
Storage/Transport Temperature
Range/ Humidity - 20°C to + 70°C, 10% RH to 95% RH
Operating and Storage/Transport
Atmospheric Pressure 700 hPa to 1,060 hPa
Protection against electric shock
- Classified as Type
- Type BF applied parts: Mouthpiece, nosepiece, masks
Degree of safety in the presence of flammable anesthetics or oxygen
No AP/APG (not suitable for use in the presence of flammable anesthetics or
oxygen)
Mode of operation Continuous
IP21 Protection against harmful ingress of water is Ordinary.