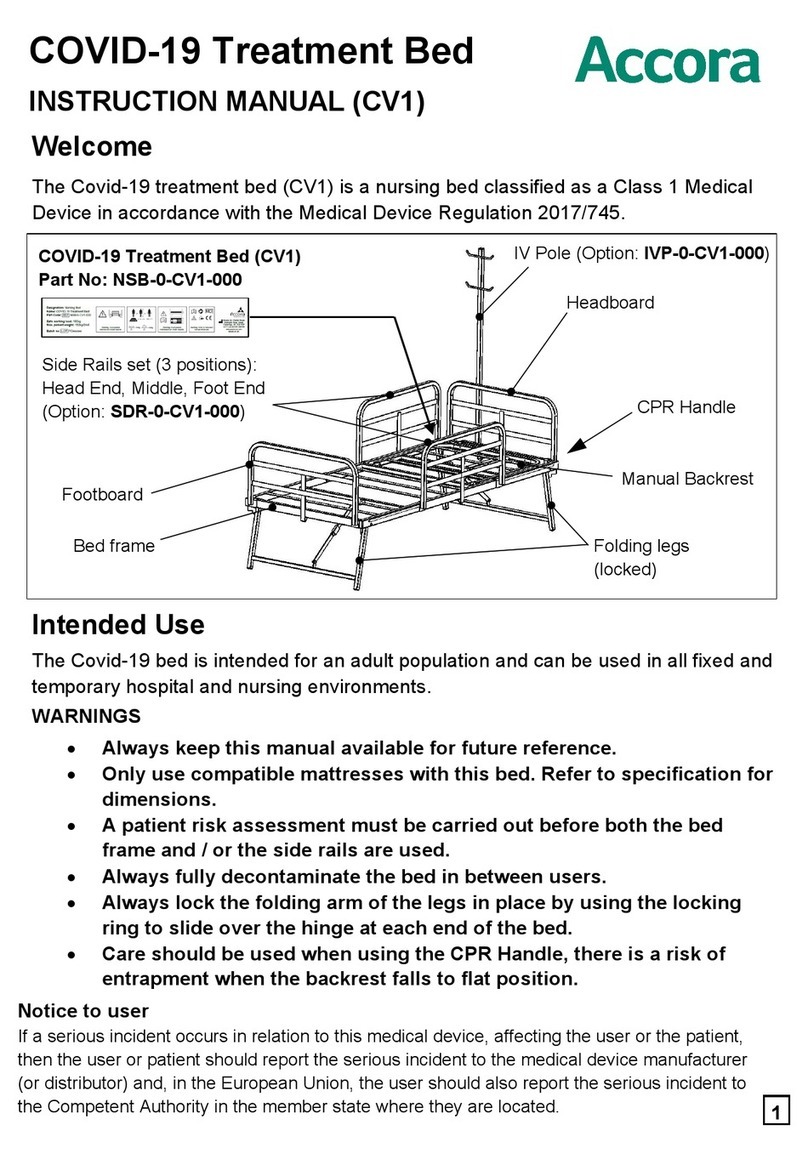
2
Contents
Title Page
Welcome 2
1. General Safety Instructions 3
2. Intended use 4
3. Clinical Applications 4
4. Model Numbers and Compatibility 5
5. Technical Specification 5
6. Before Use 6
7. Fitting CushionAir Cells 6
8. Using the Battery Powered Pump 9
9. Using the Mains Powered Pump 12
10. Maintenance 13
11. Cleaning 14
12. Storage and Transport 14
13. Product Disposal 14
14. Guarantee 14
15. Table of Symbols 15
16. Contact Details 15
Welcome
Dear Customer,
Thank you for purchasing an Accora healthcare
product. Before operating the CushionAir system,
you must read and understand all the instructions
in this manual. All actions and handling of the
product must be performed in accordance with
the instructions in this manual.
Please ensure that the manual is available to
users and operators throughout the product
service life.
If you need further information, please contact
us. See section 16 for region specific contact
details.
General
This accessory is classified as a Class 1 Medical
Device in accordance with the Medical Device
Regulation 2017/745.
Notice to User
If a serious incident occurs in relation to this
medical device, affecting the user or the patient,
then the user or patient should report the serious
incident to the medical device manufacturer (or
distributor) and, in the European Union, the user
should also report the serious incident to the
Competent Authority in the member state where
they are located.
Accora Ltd, 38 Main Street,
Swords, Co. Dublin, Ireland, K67 E0A2
T: +353 (0)1 695 0614
Design Policy and Copyright
® And ™ are trademarks belonging to Accora
Ltd unless otherwise stated. As our policy is one of
continuous improvement, we reserve the right to
modify designs without prior notice. © Accora Ltd
2021.