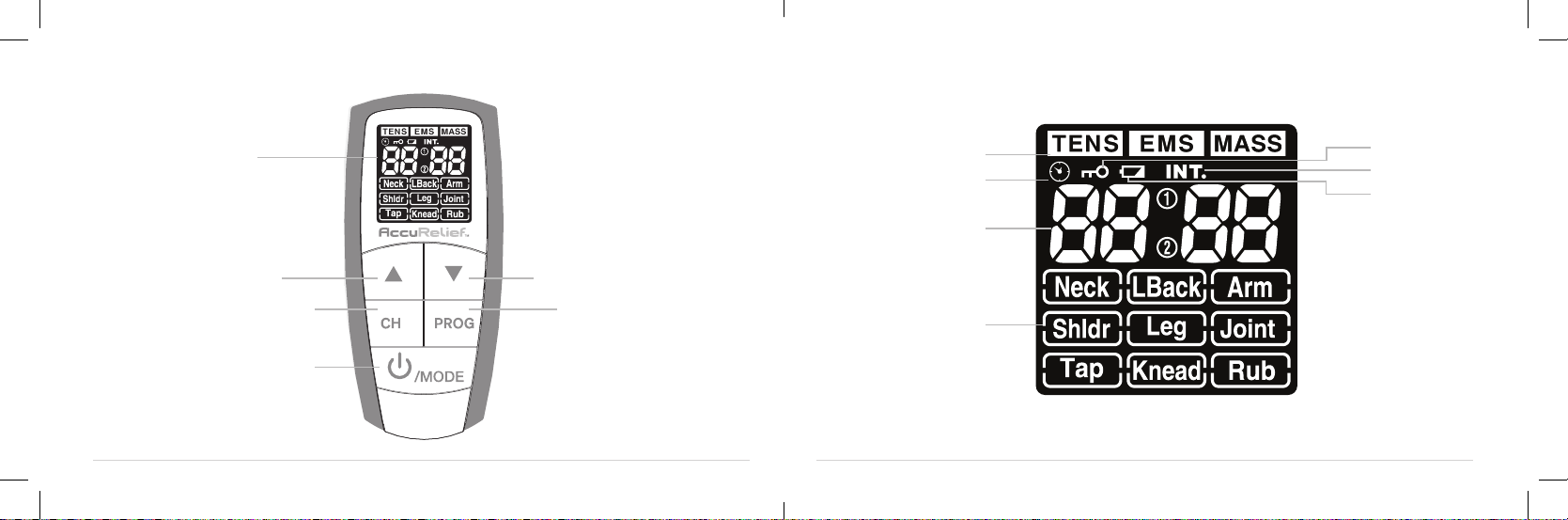
8 9
stimulation during pregnancy has not been
established.
nChildren or infants. The device has not been
evaluated for pediatric use.
nPersons incapable of expressing their
thoughts or intentions.
DO NOT USE THIS DEVICE DURING
THESE ACTIVITIES:
nWhen in the bath or shower.
nWhile sleeping.
nWhile driving, operating machinery, or
during any activity in which electrical
stimulation can put you at risk for injury.
WARNING
PAIN MANAGEMENT WARNINGS
nIf you have had medical or physical
treatment for your pain, consult with your
physician before using this device.
WARNING DO NOT USE
THIS DEVICE UNDER THESE
CONDITIONS:
nOn open wounds or rashes, or over swollen,
red, infected, or inamed areas or skin
eruptions (e.g., phlebitis, thrombophlebitis,
varicose veins); or on top of, or in proximity
to, cancerous lesions.
nOver areas of skin that lack normal sensation.
nWhile the user is connected to high-
frequency surgical equipment. It may
cause burn injuries on the skin under the
electrodes, as well as problems with the
stimulator.
nIn the vicinity of shortwave or microwave
therapy equipment. This may affect the
output power of the product.
DO NOT USE ON THESE INDIVIDUALS:
nPregnant women. The safety of electrical
nIf your pain does not improve, becomes
seriously chronic or severe, or continues for
more than ve days, stop using the device
and consult with your physician.
nThe mere existence of pain functions as
a very important warning telling us that
something is wrong. Therefore, if you
suffer from any serious illness, consult
your physician in order to conrm that it is
advisable for you to use this product.
WARNINGS AND PRECAUTIONS
REGARDING THE Electrode pads
nApply pads to normal, healthy, clean, dry
skin (of adult patients). It may otherwise
disrupt the healing process.
nIf you experience any skin irritation or
redness after a session, DO NOT continue
therapy on that area of the skin.
nPads SHOULD NOT touch each other when
placed onto your skin.
nPads MUST be placed at least 2 inches
apart, no more than 6 inches apart.
DANGER
NEVER APPLY THE PADS TO
nThe head or any area of the face.
nThe side of the neck (on the carotid
sinus) or any area of the throat
(front of the neck). This could cause
severe muscle spasms resulting in
closure of the airway, difculty in
breathing, or adverse effects on
heart rhythm or blood pressure.
nBoth sides of the thorax
simultaneously or across your
chest. The introduction of electrical
current may cause rhythm
disturbances which could be lethal.
nSpine or backbone.
nBoth feet, legs, arms or hands at
the same time.
ACRL3100_Manual_03.indd 8-9 10/31/18 4:41 PM