iv | ACIST RXi System User’s Guide 901700-001,01 2019-09 English
Perform Pa Scaling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27
5 Basic Operating Procedures 29
Before Using the ACIST RXi® System . . . . . . . . . . . . . . . . . . . . . . . . . . .29
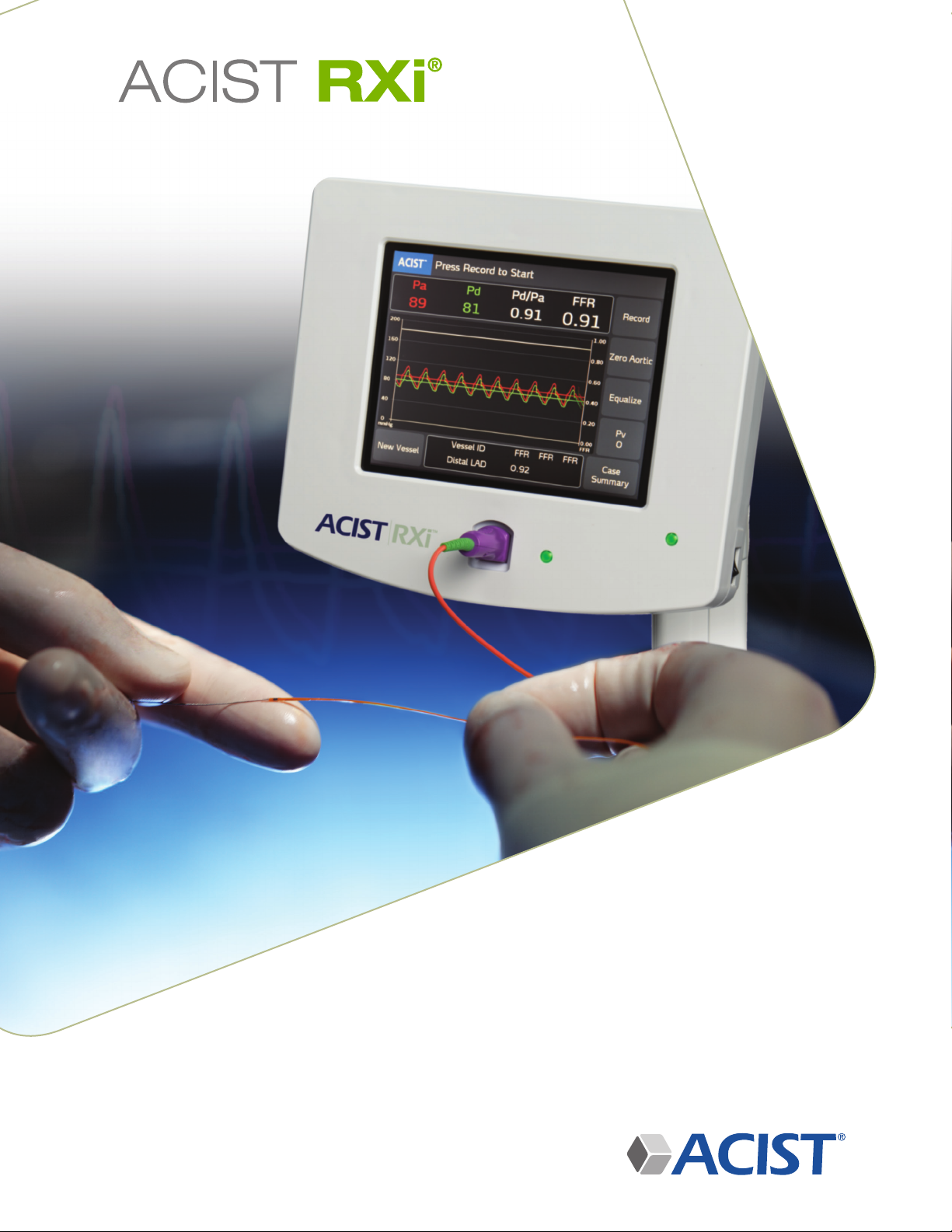
Start the ACIST RXi® System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
Power On . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
Verify that the Aortic Pressure is Zero. . . . . . . . . . . . . . . . . . . . . . . . . . .31
Zero Aortic Pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Identify the Patient ID and the Vessel ID for the Case . . . . . . . . . . . . . . . . . . . . 32
Patient ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Vessel ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Unpack and Prepare the Navvus® MicroCatheter . . . . . . . . . . . . . . . . . . . . . . 33
Preparation Needed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Deliver the Navvus® MicroCatheter, Equalize Signals, and Enter Venous Pressure . . . . . . .37
Equalize Signals and Adjust Pv . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Detection of Broken Navvus® MicroCatheter. . . . . . . . . . . . . . . . . . . . . . . .40
Record the FFR for the First Vessel . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
Record . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Record the FFR for Another Vessel . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Review the Case Summary and Export Data . . . . . . . . . . . . . . . . . . . . . . . .44
New Vessel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Case Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Shutting Down the ACIST RXi® System . . . . . . . . . . . . . . . . . . . . . . . . . .45
Power O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
6 System Information and Settings 47
About the ACIST Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
System Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
System Software Version, Date, and Time . . . . . . . . . . . . . . . . . . . . . . .49
System Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50
Change the Language Displayed in the System Software. . . . . . . . . . . . . . . . .51
Adjust the Date Setting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
Adjust the Time Setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
Enter or Modify the Facility Name . . . . . . . . . . . . . . . . . . . . . . . . . . .54
Lab ID, Mobile or Stationary. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
Enter or Modify a Lab ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .56