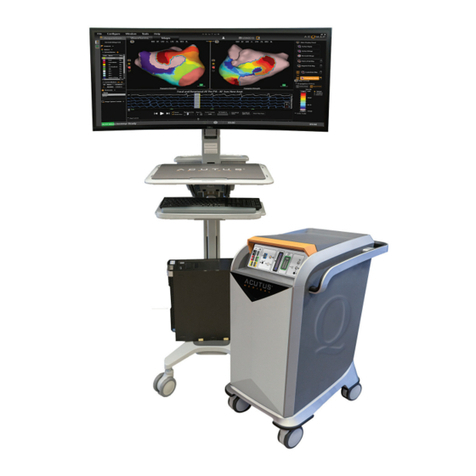
Acutus Medical Qubic Force User manual

Qubic Force
Device for visualization of contact force
of the catheter tip on the cardiac wall
EP // External devices // Technical Manual

Table of Contents
1Introduction 1
About the Device 1
About this Technical Manual 2
2Safety during Use 4
Required Expertise 4
General Safety Warnings 7
Operating Conditions 9
Maintenance, Care and Disposal 10
3Device Handling 15
Device Overview 15
Setting up the Device 19
Connections and Cables 20
Switching On and O 25
Keys on the Device 26
4Using the Software 28
The Main View 28
The Status Bar 29
The Numerical Display 31
The Graphic Display 32
The Trend Display 34
The Settings View 35
5Appendix 38
Technical Data 38
Parameter Values 40
Accessories 41
Country-Related Information 42
Legend for the Label 43
6Directories 45
Index 45

1
Introduction
1Introduction
About the Device
General description
Qubic Force is used with the AcQBlate® FORCE ablation catheter, a
compatible radiofrequency (RF) generator, and an external monitor. Qubic
Force is a device for visualization of the contact force of the ablation
catheter tip on the cardiac wall during an electrophysiological study in
cardiac catheter laboratories with or without cardiac radiofrequency (RF)
ablation. An external monitor is placed in a position easily visible to the
user and connected to the Qubic Force. The contact force is displayed
which allows the user to monitor the contact force of the ablation catheter
tip on the cardiac wall, establish proper contact on the cardiac wall, and
inuence lesion formation.
Intended medical use
The relevant cardiology association guidelines do not contain a medical
indication for the visualization of the contact force of the catheter tip on
the cardiac wall and therefore contain no indication for the use of Qubic
Force.
Qubic Force is not required to perform an electrophysiological study
in cardiac catheter laboratories with or without cardiac radiofrequency
ablation, but this device does provide important information to the user,
for example for the assessment of lesion formation and optimization of
ablation parameters.
Contraindications
There are no specic contraindications for the use of Qubic Force. For
information on contraindications for the ablation catheter and the RF
generator, please consult their technical manuals.
Patient group
Use of Qubic Force is indicated for all patients subjected to a therapeutic
electrophysiological study. For studies using Qubic Force, there are no
restrictions in terms of the age, sex, weight, state of health, nationality, or
condition of the patient.

2
Introduction
Compatible RF generators
The following RF generators are compatible with Qubic Force:
• BIOTRONIK: Qubic RF
• Stockert: EP-Shuttle
• Biosense Webster: SMARTABLATE™ HF Generator (manufacturer:
Stockert)
• St. Jude Medical: IBI-1500 T11
• Medtronic: Atakr II
• Osykpa: HAT 300 Smart
About this Technical Manual
Objective
This technical manual provides all the safety information required to use
the device.
The following topics are covered in this manual:
• Device startup
• Device handling
• Using the software
Target group
This technical manual is intended for cardiologists, electrophysiologists and
cardiac surgeons possessing knowledge in the following areas:
• Catheterization procedures
• Procedures for ablating the intracardiac stimulation and conduction
systems
This technical manual is also intended for clinical and technical assistants
possessing expertise in handling devices in cardiac catheter laboratories.
Additional required expertise is:
• Basic medical knowledge of the examination method employed
• Ability to work with a PC
• Ability to use software-controlled medical devices

3
Introduction
Other Technical Manuals
The following additional technical manuals must be followed to ensure the
safe and correct use of the device:
• Technical manuals for other system components in the cardiac cath-
eter laboratory, not supplied with the Qubic Force (e.g., AcQBlate®
FORCE ablation catheter, RF generator, lab monitoring system, and
external monitor)
• Technical manuals for the intended catheters, indierent electrodes,
patient cables, and adapters
• Technical manuals for other designated accessories

4
Safety during Use
2Safety during Use
Required Expertise
Required expertise
Qubic Force is intended for use by cardiologists, electrophysiologists,
cardiac surgeons, and clinical and technical assistants specialized in
handling devices in cardiac catheter laboratories and trained in handling
the Qubic Force. In addition to having basic medical knowledge, the user
must be thoroughly familiar with the electrophysiology of the heart,
catheterization procedures, and the method of ablating the intracardiac
stimulation and conduction system.
Only trained and qualied medical personnel with this knowledge can
properly operate the device.
Note: Please note that in principle there is a risk of cardiac wall
perforation during a cardiac radio frequency ablation and that this
cannot entirely be excluded despite the use of Qubic Force. Therefore,
take any measures to minimize this risk as much as possible.
Electromagnetic Interference
Possible electromagnetic interference
This device is protected against electromagnetic interference and
electro- static discharges in the specialized environment of a cardiac
catheter laboratory containing high-frequency surgical instruments and
X-Ray devices. At the same time, the emitted interference is reduced to a
minimum.
The device thus fullls the requirements of EN 60601-1-2 as they apply to
CISPR 11 class A in relation to both interference emitted and resistance to
interference. The following norms do not apply here:
• IEC 61000-3-2
Harmonic distortion (harmonic currents in the mains supply)
• IEC 61000-3-3
Voltage uctuations and icker in the mains supply
5
Safety during Use
The following tests were performed according to IEC 60601-1-2: 2014:
Section of
IEC 60601-
1-2:2014
Test Test level
7.1 EN 55011 (CISPR 11)
Conducted
interference
emissions
• Group 1
• Class A
EN 55011 (CISPR 11)
Radiated emissions
8.9 IEC 61000-4-2
Electrostatic
discharge (ESD)
• ±8 kV contact discharge
• ±15 kV air discharge
8.9 / 8.10 IEC 61000-4-3
Electromagnetic
elds
• Modulation: 1 kHz
• 3 V/m, 80 MHz – 2.7 GHz
• Limits for RF communication
equipment per Table 9 in IEC
60601-1-2 (9-28 V/m)
8.9 IEC 61000-4-4
Transient conducted
surge voltages (EFT,
bursts)
• ± 2kV mains supply
• ± 1kV signal line
IEC 61000-4-5
Surge voltage waves
on supply lines
• ± 2 kV common mode
• ± 1 kV common mode
IEC 61000-4-6
Conducted
radio- frequency
interference
• Modulation: 1 kHz
• 3 V
• 6 V in ISM bands
IEC 61000-4-8
Power frequency
magnetic elds
• 30 A/m
• 50/60 Hz
IEC 61000-4-11
Voltage uctuations
and interruptions in
supply voltage

6
Safety during Use
Even when, as pointed out above, the device complies with the
requirements of EN 60601-1-2, strong electromagnetic disturbances may
occur in the immediate vicinity of electrical motors, high-voltage power
lines, PCs, monitors and other – perhaps defective – electrical devices
which may cause the Tare key to be triggered unintentionally and may
sometimes impair the functioning of the device.
This kind of device malfunction should be considered as a possible cause if
the following is observed:
• The values displayed for contact force and application angle are set to
zero with the AcQBlate®FORCE ablation catheter connected, as long as
the Tare key has not been pressed.
• The device displays other inexplicable behavior.
Correct operation of the device can be restored with the following
miscellaneous measures:
• Switch o electronic device generating the disturbance.
• Remove the source of interference from the device.
• Switch the device on and o or break the electrical connection be-
tween the device and the source of the interference if this can be done
safely.
If the interference continues, contact Acutus Medical immediately.
WARNING
Risk of electromagnetic interference through the use of unau-
thorized accessories
The use of accessories, transducers or cables not listed by Acutus Medical
or of accessories other than those specied by Acutus Medical, can
produce elevated electromagnetic emissions or cause degradation in the
device’s resistance to electromagnetic interference. Such eects can lead
to the faulty operation of the device.
• Use only accessories authorized by Acutus Medical

7
Safety during Use
Even when, as pointed out above, the device complies with the
requirements of EN 60601-1-2, strong electromagnetic disturbances may
occur in the immediate vicinity of electrical motors, high-voltage power
lines, PCs, monitors and other – perhaps defective – electrical devices
which may cause the Tare key to be triggered unintentionally and may
sometimes impair the functioning of the device.
This kind of device malfunction should be considered as a possible cause if
the following is observed:
• The values displayed for contact force and application angle are set to
zero with the AcQBlate®FORCE ablation catheter connected, as long as
the Tare key has not been pressed.
• The device displays other inexplicable behavior.
Correct operation of the device can be restored with the following
miscellaneous measures:
• Switch o electronic device generating the disturbance.
• Remove the source of interference from the device.
• Switch the device on and o or break the electrical connection be-
tween the device and the source of the interference if this can be done
safely.
If the interference continues, contact Acutus Medical immediately.
WARNING
Risk of electromagnetic interference through the use of unau-
thorized accessories
The use of accessories, transducers or cables not listed by Acutus Medical
or of accessories other than those specied by Acutus Medical, can
produce elevated electromagnetic emissions or cause degradation in the
device’s resistance to electromagnetic interference. Such eects can lead
to the faulty operation of the device.
• Use only accessories authorized by Acutus Medical
WARNING
Risk of electromagnetic interference through the use of porta-
ble RF communication devices
If portable RF communication devices (including peripheral devices
such as antenna cables and external antennae) are operated closer than
30 cm (12 inches) from this device, this can result in a reduction in its
performance. This applies even if using the cables specied by Acutus
Medical, Inc.
• When operating portable RF communication devices (including
peripheral devices such as antenna cables and external antennae),
keep such devices at a distance of at least 30 cm (12 inches) from
this device.
General Safety Warnings
Risks of improper handling
Disregarding the safety warnings can endanger the patient, the sta and
the equipment.
Note: Failure to observe the safety warnings voids all damage claims and
manufacturer liability.
The following dangers can, for example, arise in the event of improper use:
• Failure of important device functions
• Personal endangerment due to electrical impact
Changes not permitted
Only the manufacturer or a party expressly authorized by Acutus Medical
may perform corrective maintenance, enhancements or modications to
the device.
Replacement parts and accessories
Use only accessories authorized by Acutus Medical. Using any other parts
voids liability for any consequences, as well as the product guarantee and
warranty.
RF accessories
Use only RF accessories certied according to Standard IEC 60601-2-2.

8
Safety during Use
Defective devices
Do not use defective or damaged devices.
Physician supervision
The device may only be used under the constant supervision of a physician.
The patient must be monitored at all times using an external surface ECG
with rate control.
Patient observation
Ensure that patients are individually observed over a suitable period
of time in order to monitor the compatibility and eectiveness of the
electrophysiological therapy.
Emergency equipment
During an examination, keep resuscitation equipment (e.g., cardiac
debrillator, external pacemaker) available and ready for use at all times in
order to perform life-supporting measures immediately in the event of an
emergency.
Liquids
Never use a damp or wet device. Protect the device from accidental
ingression of uids (e.g. infusion uids).
If the device becomes wet, immediately unplug and stop using the device.
Contact Acutus Medical for testing and, if necessary, repair of the device.
Electrostatic potentials
Ensure that electrostatic potentials between medical sta and patients
are balanced. Before handling the device, the electrostatic potential
between the physician or medical sta and the patient must be balanced
by touching the patient at a point as far away from the catheters or leads as
possible.
Leakage currents
Avoid leakage currents between all connected devices. Such leakage
currents can cause lethal arrhythmias.
Potential equalization cables must be attached to all connected
components, if present.
Before initial commissioning, check and document all device combinations.
National and international directives concerning the use of electromedical
devices also apply to patient cables.

9
Safety during Use
Touching contacts on cables and catheters
Do not touch the contacts on the patient cable or the catheters. The device
has electrical contact with the patient’s heart and blood via the implanted
catheters. Touching the contacts on the patient cable or catheters could
expose the patient’s heart to dangerous electrical currents.
Defibrillation
When connected with the approved patient cable, the device is
debrillation protected. However, damage cannot be ruled out in all
circumstances.
Following a debrillation, the recovery time can take up to 10 seconds
until the device is ready for use again. Check all functions of the device,
following a debrillation. During debrillation, do not touch the patient,
the device the patient is connected to, or the attached accessories.
Otherwise, there is a danger that you may suer an electrical shock.
Risk of infection
Contaminated devices can lead to infection. Clean and disinfect the device
on a regular basis. Refer to the cleaning instructions for all other system
components.
Operating Conditions
Storage and transportation
If the packaging is damaged, please contact Acutus Medical immediately.
Do not put the device into operation.
The ambient conditions for shipping and storage are:
Temperature 0°C ... +50°C
Relative humidity 30% ... 75%, no condensation
Atmospheric pressure 700 ... 1060 hPa
Operating conditions
Note: After transporting the equipment from a cold to a warm area,
condensation may form, particularly on metal parts of the device, and
damage the electronics.
• After transport, wait approximately 2 hours until the device has
reached room temperature and the condensation has dried up
before using the system.

10
Safety during Use
WARNING
Risk of electromagnetic interference
The use of this device close to or in direct contact with other devices
should be avoided, as this may lead to the device operating incorrectly.
• Where usage in such a manner is unavoidable, you should monitor
this device and the device or devices being used together with it in
order to check that they are all working correctly.
WARNING
Risk of electromagnetic interference through the use of porta-
ble RF communication devices
If portable RF communication devices (including peripheral devices
such as antenna cables and external antennae) are operated closer than
30 cm (12 inches) from this device, this can result in a reduction in its
performance. This observation also applies even to the specied cable.
• When operating portable RF communication devices (including
peripheral devices such as antenna cables and external antennae),
keep such devices at a distance of at least 30 cm (12 inches) from
this device.
Only operate the device in rooms that fulll the following conditions:
• No danger of explosion
• Suitable for medical purposes
• Class I power outlet with protective conductor connection
Place the device in a position protected from spray water. Place the device
on a at, dry surface. Place the device in a position where it cannot slip,
even with cables connected, nor be touched by the patient, and so that you
can pull the power plug out of the device at any time. Make sure that the
ventilation slots remain unobstructed. The device cannot be sterilized and
therefore must not be operated in sterile areas.
The ambient conditions for operation are:
Temperature +10°C ... +40°C
Relative humidity 30% ... 75%, no condensation
Atmospheric pressure 700 ... 1060 hPa
Operation at altitudes Up to 2000 m AMSL

11
Safety during Use
WARNING
Risk of electromagnetic interference
The use of this device close to or in direct contact with other devices
should be avoided, as this may lead to the device operating incorrectly.
• Where usage in such a manner is unavoidable, you should monitor
this device and the device or devices being used together with it in
order to check that they are all working correctly.
WARNING
Risk of electromagnetic interference through the use of porta-
ble RF communication devices
If portable RF communication devices (including peripheral devices
such as antenna cables and external antennae) are operated closer than
30 cm (12 inches) from this device, this can result in a reduction in its
performance. This observation also applies even to the specied cable.
• When operating portable RF communication devices (including
peripheral devices such as antenna cables and external antennae),
keep such devices at a distance of at least 30 cm (12 inches) from
this device.
Only operate the device in rooms that fulll the following conditions:
• No danger of explosion
• Suitable for medical purposes
• Class I power outlet with protective conductor connection
Place the device in a position protected from spray water. Place the device
on a at, dry surface. Place the device in a position where it cannot slip,
even with cables connected, nor be touched by the patient, and so that you
can pull the power plug out of the device at any time. Make sure that the
ventilation slots remain unobstructed. The device cannot be sterilized and
therefore must not be operated in sterile areas.
The ambient conditions for operation are:
Temperature +10°C ... +40°C
Relative humidity 30% ... 75%, no condensation
Atmospheric pressure 700 ... 1060 hPa
Operation at altitudes Up to 2000 m AMSL
Power supply
The device is operated via the AC voltage (100 to 240 V at 50 / 60 Hz) of a
room used for medical purposes.
CAUTION
Possibility of electric shock
To avoid the risk of electric shock, connect the device only to a power
supply tted with a PE conductor.
The electrical port must fulll the following conditions:
• The power outlet fullls at least the requirements of IEC 60364-7-
710:2002 group 2.
• The device cable feeds directly into a permanently installed socket. No
portable multiple socket outlets may be used.
• When used in combination with other devices, no portable multiple
socket outlets should be used.
• Only power cords which are suitable for medical devices can be used,
such as power cords from Acutus Medical or equivalent power cords
labeled H05VV 3 x 0.75 mm, H05VV 3 x 1 mm, or SJT AWG18.
To disconnect Qubic Force from the mains supply, pull the power plug out
of the device.
Cable and plug connections
WARNING
Allergic reaction
The cable material may trigger allergic reactions in extremely rare cases.
• Prevent the cable from contacting the skin or wounds.
• Replace any cable that shows even slight damage.
• Lay all cables between the patient and the device, as well as within the
measuring apparatus, in such a way that they pose no danger of trip-
ping and that any tensile forces that may occur can be safely buered.
• Ensure that the contacts of all connector ports and connectors are
clean. Soiled contacts can lead to signal distortions, and thus to false
diagnoses.
• Ensure that there is no condensation on the plugs or in the connector
ports. If condensation is present, dry it before use.
• Do not force the plugs into the connector ports. Do not pull on the ca-
ble when disconnecting the plugs. Rather, release the lock on the plug.

12
Safety during Use
Maintenance, Care and Disposal
General information
Note: Note the following points before cleaning and disinfecting:
• Disconnect the power plug before cleaning and disinfecting the
device surfaces.
• Let cleaning and disinfection agents evaporate before operating the
device.
• Do not use any strong and abrasive cleaning agents or organic
solvents such as ether or benzine, as they corrode the surface of the
device.
Cleaning and disinfecting
• Use lint-free, soft cloths.
• Clean the housing with a damp cloth and mild soap solution or 70%
isopropanol.
• Disinfect with alcohol-based agents such as Aerodesin 2000.
Sterilization
• The device is not sterile and cannot be sterilized.
CAUTION
Infection of the patient from operation of the non-sterile device
Qubic Force is not sterile and cannot be sterilized. If, during the ablation
therapy of the patient, the physician operates the device at the same
time, infection of the patient can result.
• During ablation therapy, do not operate the device at the same time.

13
Safety during Use
Test before each use
• A test of the device and the approved accessories should be per-
formed prior to each use. This test consists of the following visual
inspections and a simple functional test:
– Inspect the housing for mechanical damage, dents, loose
parts, cracks, etc.
– Inspect cables and connection areas to ensure proper
insulation, the absence of breaks, etc.
– Inspect the labeling for legibility.
– Perform a simple electrical function test by switching on
the device.
– An internal function test is performed automatically.
– If no error message appears, then no errors were found
and the device can be used.
– Inspect the displays (e.g., display of characters and lan-
guage).
Inspection
The inspection consists of the regular safety inspection according to
medical device standards. This ensures the safety of the device.
• Inspections should be performed:
– If malfunctions are suspected
– Once a year
• The inspection can be performed by Acutus Medical.
• The inspection must conform to the manufacturer’s specications.
These are available upon request. The specications list all necessary
test steps and the necessary equipment.
• The instructions for performing the inspection are directed at people
whose education, knowledge, and experience obtained through prac-
tical work provide the basis for proper execution.
Fuse replacement
The fuses are located above the power cord port in a fuse holder.
Step Action
1 Turn the device o and unplug the power cord.
2 Use a suitable tool to pull the fuse holder out.
3 Replace the old fuses with new ones of the same type.
4 Re-insert the fuse holder. Ensure that it locks securely in place.

14
Safety during Use
Note: Defective fuses can indicate a technical defect in the device.
Conduct an inspection after changing fuses and before resuming
operation of the device.
Disposal
The symbol on the type plate, a crossed out garbage can, indicates that
the device must be disposed of in accordance with the European Directive
2012/ 19/EU on waste electrical and electronic equipment (WEEE 2). If
the device is not disposed of in an environmentally friendly manner, it
will result in environmental pollution as this device contains materials
which must be disposed of in accordance with environmental protection
requirements (e.g., WEEE, RoHS, REACH). Return devices that are no longer
in use to Acutus Medical.
Disposal of cables
Note: Cables that are to be disposed of must be treated as medical
waste, in accordance with environmental regulations, if they have been
in contact with blood
Non-contaminated cables must be disposed of in accordance with
Directive 2012/19/EU on waste electrical and electronic equipment (WEEE
2) or in accordance with the regulations applicable locally.

15
Device Handling
3Device Handling
Device Overview
Front view
1
2
3
4
5
6
7
8
9
Explanation of items
Item Description
1 Tare key
• Sets the displayed values for contact force and the angle at
which the ablation catheter is applied to the cardiac wall to
zero
2 Marker key
• Marks the current values in the log le for the current proce-
dure and stores a current screenshot
• Transfers the log le for the current procedure and the
stored screenshots to a USB ash memory stick
3 On/o key
• For switching the device on/o

16
Device Handling
4 Ventilation slots
• To protect the device from overheating
5 USB port
• To connect a mouse, keyboard or USB ash memory stick
without an independent power supply
6 Redel port for generator
• For connecting a compatible RF generator using the corre-
sponding patient cable
7 Redel port for ablation catheter
• For connecting the electrical plug of the ablation catheter
using patient cable PK-147
8 Optical port for ablation catheter
• For connecting the optical plug of the ablation catheter
9 On/o light indicator (LED)
• Lights up green when the device is switched on

17
Device Handling
Rear view of device
10
11
12
13
14
15
16
Explanation of items
Item Description
10 Redel port for expansion
• General, analog connection for expansions
(No use of this port is planned at present. Consult Acutus
Medical.)
11 Ventilation slots
• To protect the device from overheating
12 Binary interface 1 (RS-232 port)
• General, serial connection for expansions
(No use of this port is planned at present. Consult Acutus
Medical.)
13 Binary interface 2 (RS-232 port)
• General, serial connection for expansions
(No use of this port is planned at present. Consult Acutus
Medical.)
14 Power cord port and device fuse
• For connecting the power cord
15 Ethernet port (not suitable for network connection)
• General, digital connection for expansions
(No use of this port is planned at present. Consult Acutus
Medical.)
16 Monitor port
• To connect a monitor

18
Device Handling
Symbols on the device
Explanation of symbols
Symbol Description
On/o light indicator
Tare
Marker
Warning of invisible intense light from an SLED
Since this light corresponds to laser class 1, this
optical port poses no risk to the user or
patient.
Ablation catheter
Type CF applied part, debrillation protected
USB port
Radiofrequency unit of the RF generator
Follow the Instructions for Use
Table of contents
Other Acutus Medical Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual