Adaltis NEXgen User manual

Read this operator’s manual carefully before using NEXgen
U
SER
'
S
M
ANUAL

NEXgen User’s Manual
2
T
ABLE OF CONTENTS
1.
Introduction ................................................................................................................................................................ 5
1.1
Identification data ............................................................................................................................................. 5
1.2
Copyright ........................................................................................................................................................... 6
1.3
Conformity to International and European regulations .................................................................................... 6
1.4
Using the manual .............................................................................................................................................. 6
1.5
Symbols ............................................................................................................................................................. 6
1.6
Intended use and general description .............................................................................................................. 7
1.6.1
Intended use ................................................................................................................................................. 7
1.6.2
General description....................................................................................................................................... 7
1.7
Environmental protection instructions ........................................................................................................... 10
1.7.1
Disposal precautions ................................................................................................................................... 10
1.7.2
Decontamination ........................................................................................................................................ 11
1.8
Package contents ............................................................................................................................................ 12
2.
General information ................................................................................................................................................. 14
2.1
Technical specifications ................................................................................................................................... 14
2.1.1
Samples rack ............................................................................................................................................... 14
2.1.2
Disposables rack.......................................................................................................................................... 15
2.1.3
Used tips case ............................................................................................................................................. 16
2.1.4
Reagents rack .............................................................................................................................................. 17
2.1.5
Microplates holder ...................................................................................................................................... 18
2.1.6
IFA slides rack .............................................................................................................................................. 18
2.1.7
Reading system ........................................................................................................................................... 19
2.1.8
Barcode reader ........................................................................................................................................... 20
2.1.9
Barrier sensors ............................................................................................................................................ 21
2.1.10
Microplate robotic arm .......................................................................................................................... 22
2.1.11
Incubators ............................................................................................................................................... 22
2.1.12
Shaker-incubator group optional......................................................................................................... 23
2.1.13
Pipetting system ..................................................................................................................................... 24
2.1.14
Washing system ...................................................................................................................................... 25
2.1.15
Workstation minimum requirements ..................................................................................................... 28
2.1.16
Operating System specifications ............................................................................................................ 28
2.1.17
Application software specifications ........................................................................................................ 28
2.1.18
Sizes ........................................................................................................................................................ 28
2.1.19
Environmental requirements for operation ........................................................................................... 28
2.1.20
Power requirements ............................................................................................................................... 29
2.1.21
Consumption .......................................................................................................................................... 29

NEXgen User’s Manual
3
2.1.22
Connections ............................................................................................................................................ 29
2.2
Precautions and operating limitations ............................................................................................................ 29
2.2.1
General precautions.................................................................................................................................... 29
2.2.2
Health precautions ...................................................................................................................................... 29
2.2.3
Electric safety .............................................................................................................................................. 30
2.2.4
Environmental requirements ...................................................................................................................... 31
2.2.5
Waste disposal ............................................................................................................................................ 31
2.3
Main information for installation by the operator .......................................................................................... 31
3.
Operating procedure ................................................................................................................................................ 34
3.1
Before using the instrument ........................................................................................................................... 34
3.1.1
Safety recommendations ............................................................................................................................ 34
3.1.2
Treatment of samples ................................................................................................................................. 34
3.1.3
Reagents and consumables ........................................................................................................................ 35
3.2
Before programming an assay ........................................................................................................................ 36
3.3
Session programming, running and results ..................................................................................................... 36
3.3.1
Startup screen ............................................................................................................................................. 37
3.3.2
Self-test report ............................................................................................................................................ 38
3.3.3
Main Menu.................................................................................................................................................. 40
3.3.4
Load Session ................................................................................................................................................ 40
3.3.5
Select Tests ................................................................................................................................................. 42
3.3.6
Load samples .............................................................................................................................................. 43
3.3.7
Load reagents.............................................................................................................................................. 66
3.3.8
Load disposables ......................................................................................................................................... 73
3.3.9
Load plates .................................................................................................................................................. 77
3.3.10
Load tanks............................................................................................................................................... 83
3.3.11
Run session ............................................................................................................................................. 86
3.4
End of work ..................................................................................................................................................... 96
3.4.1
Clean the washing system ........................................................................................................................... 97
3.4.2
Empty the waste liquids tank ...................................................................................................................... 97
3.5
Archive .......................................................................................................................................................... 100
3.5.1
Results to be printed ................................................................................................................................. 101
3.5.2
Archived results ........................................................................................................................................ 102
3.6
Self-test report .............................................................................................................................................. 102
3.7
Maintenance ................................................................................................................................................. 103
3.8
System setup ................................................................................................................................................. 105
3.8.1
Resource page ........................................................................................................................................... 105
3.8.2
Operation page ......................................................................................................................................... 106

NEXgen User’s Manual
4
3.8.3
Report layout page ................................................................................................................................... 108
3.8.4
Run page ................................................................................................................................................... 109
3.8.5
Service page .............................................................................................................................................. 111
4.
Maintenance ........................................................................................................................................................... 112
4.1
General rules ................................................................................................................................................. 112
4.2
Responsibilities .............................................................................................................................................. 113
4.3
Daily maintenance procedure ....................................................................................................................... 113
4.4
Weekly maintenance procedure ................................................................................................................... 113
4.4.1
Washer check ............................................................................................................................................ 113
4.5
Monthly maintenance procedure ................................................................................................................. 114
5.
Troubleshooting guide ............................................................................................................................................ 116
5.1
General criteria ............................................................................................................................................. 116
5.2
Self-test system ............................................................................................................................................. 116
5.3
Classification of causes of main malfunctions ............................................................................................... 116
5.3.1
Malfunction caused by a fault in the power supply .................................................................................. 116
5.3.2
Error messages and anomalies about software that can occur during Computer startup and OpenLAB
program run ............................................................................................................................................................ 117
5.3.3
Error messages that can occur during instrument self-test ...................................................................... 117
5.3.4
Error messages due to pipetting system .................................................................................................. 118
5.3.5
Incubators malfunctions ........................................................................................................................... 118
5.3.6
Moving parts malfunctions ....................................................................................................................... 119
5.3.7
Reading system malfunctions ................................................................................................................... 119
5.3.8
Washing system malfunctions .................................................................................................................. 119
5.4
Error messages due to an incorrect session loading ..................................................................................... 119
5.5
Error messages and actions to be taken by the operator ............................................................................. 120
5.6
Responsibilities .............................................................................................................................................. 120

NEXgen User’s Manual
Introduction
5
1. I
NTRODUCTION
1.1 I
DENTIFICATION DATA
This is the Operator’s Manual for the NEXgen instrument, an automatic analyzer for enzyme immunoassay
kits on microplates and IFA kits on slides.
This document must be considered as an integral part of the NEXgen instrument and the information
herein must carefully be read by the operator before using the instrument.
It is strongly recommended to carefully read the content of this Manual before working on the instrument.
the operator must pay particular attention to the Intended use and general description and Precautions
and operating limitations (page 29) paragraphs content and follow the directions included in the present
Manual. In case any possible damages or problems occur, please contact the proper Technical Assistance.
The Manufacturer disclaims any responsibility for partial or unauthorized copies of the present Manual.
Manual
Manual part number
NEX2337V4
.
2
Revision
Rev.
A
Software version
4.2
Date of issue
13/10/2015
Instrument
Instrument
code
NXG
Manufacturer
Adaltis Srl
Via Durini, 27
20122 Milano (Italy)
Tel. +39-0774-5791 - Fax +39-0774-353085
www.adaltis.net

NEXgen User’s Manual
Introduction
6
1.2 C
OPYRIGHT
The Operator’s Manual is an integral part of the NEXgen instrument, its content is the exclusive property of
Adaltis Srl, all rights reserved. Any disclosure, unauthorized copy of its contents or transfer to third-party is
forbidden without having a previous license by Adaltis Srl.
1.3 C
ONFORMITY TO
I
NTERNATIONAL AND
E
UROPEAN REGULATIONS
This document is written in accordance to the UNI EN ISO 18113-3:2012 regulation.
The NEXgen instrument conforms to the 98/79 European Community Directive and subsequent
modifications.
The NEXgen instrument has also been designed in accordance to the following regulations:
Safety
CEI EN 61010
-
1 (E
D
. 20
13
-
1
0)
Electromagnetic compatibility
CEI EN 61326
-
1 (E
D
. 20
13
-
0
7
)
CEI EN 61326-2-6 (E
D
. 2014-06)
1.4 U
SING THE MANUAL
The Manufacturer recommends the operator to read every section of the present Operator’s Manual.
Particular attention must be paid to the NOTES, providing important information for a proper use of the
NEXgen instrument and to the WARNINGS, emphasizing potential risks or dangers deriving from the
instrument use.
In this present manual, the accent color term will be used to identify the blue color that is used by the
software to underline any specific element that has a particular meaning in the current context.
1.5 S
YMBOLS
The following symbols are present on the NEXgen instrument. It is important that the operator knows their
meaning in order to make a proper and safe use of the instrument.
Key to symbols on the external label on the right side of NEXgen
Instrument th
at conforms to the requirements of European Directive for in vitro
diagnostic device (98/79/CE).
In vitro diagnostic device.
Instrument that conforms to the CSA standards for Canadian and American market.
Instrument manufacture
r
date.

NEXgen User’s Manual
Introduction
7
Instrumen
t serial number.
Manufacturer data.
Key to electric and safety symbols on the different parts of NEXgen
Grounding terminal.
RAEE: Electric
-
Electronics Instrument
Separate compulsory collection of waste under
D.L. 25/07/2005 n° 151 (Italy), implementation of 2002/96/EC and 2003/108/EC
Directives.
Warning, read the Manual and pay attention to the safety symbols.
Warning, risk of electric shock.
Warning, hot surface.
Warning, biohazard.
Warning, hands prick/cut danger.
Warning, cru
sh danger.
Warning, laser beam.
1.6 I
NTENDED USE AND GENERAL DESCRIPTION
1.6.1 I
NTENDED USE
The NEXgen is an automated analyzer which comes with the integrated OpenLAB software and it is
designed to simultaneously perform, even on the same samples, ELISA tests on microplates and IFA tests on
slides.
The NEXgen automates every step of the diagnostic tests processes for which it is enabled (dilution and
dispensing of both samples and controls, reagents dispensing, incubation, shaking, washing, reading, results
processing, interpretation and archiving) to be performed quickly, accurately and precisely.
1.6.2 G
ENERAL DESCRIPTION
The NEXgen is an open system able to process both ELISA microplate and IFA slide tests designed for high
volume throughput and multiple assay applications.

NEXgen User’s Manual
Introduction
8
Image 1- NEXgen
The NEXgen is equipped with 3 robotic arms. Two of them are located in the working area, one is used for
pipetting and liquid management, and the other for the wash manifold. The third robotic arm is located
under the working area and devoted to moving the microplates holder and the IFA slides racks both in the
working area and in the reading area.
Each robotic arm has independent movements that do not interfere with the movements of the others.
This gives the NEXgen an extreme advantage for flexibility and independence of the single operations to the
benefit of the execution time of the single sessions.
The NEXgen w rking area is designed to be extremely flexible. Numerous resources configurations can be
loaded on the slots so that, as needed, many samples can be processed against one or more tests or less
samples against many tests. Up to 5 microplates can be loaded on incubators on the working area and 2
additional microplates can be loaded in a compartment equipped with incubator located under the working
area.

NEXgen User’s Manual
Introduction
9
Image 2 - W rking area
The NEXgen is able to process samples both in batch mode and in continuous loading. NEXgen’s batch
mode is able to manage up to 600 samples against one test and 192 samples against multiple tests.
Intermediate configurations depend on the number of samples and tests. By decreasing the number of
tests, the number of samples increases.
The NEXgen is also able to process up to 20 IFA slides per run. In this case, the maximum number of
samples is related to the IFA slides configuration in terms of number of wells, considering the capacity of 5
racks, each can hold 4 IFA slides. The NEXgen can also run both ELISA and IFA tests in the same session.
Image 3 - ELISA and IFA c nfigurati n
NEXgen carries out the diluti n and dispensing procedures by using a double channel pipetting system that
allows the system to dispense the liquids in an extremely short time. The advantage is to minimize the

NEXgen User’s Manual
Introduction
10
“drift” effect across the plate in addition to a reduction of the execution times of each single session. The
use of the 1000 µL and 200 µL standard disposable tips eliminates carryover. The flow sensor, together with
a modern electronics, enables the 2 pipettors to precisely and unequivocally control both the quantity of
aspirated/dispensed liquids and the detection of possible clots in the sera.
The NEXgen wash stati n, on the working area, and the incubators positioned under the 5 microplates
enable it to follow the incubation times of the single strips for each microplate as stated in the Instruction
for use. While a strip is being washed, the others are incubated until the end. This unique feature of the
NEXgen instrument, together with the high speed liquids dispensing, minimizes the “drift” effect.
NEXgen is equipped with a 8-channel manif ld that uses short dispensing needles and long aspirating
needles. Washing cycles, volume and soak time are programmable. In the case of IFA slides, the 1000 µL tip
carries out the wash buffer dispensing into each single well while the manifold carries out the aspiration.
NEXgen is equipped with 5 incubat rs located in the working area under the microplates and 2 incubat rs
located under the working area. The incubators work in a temperature range from room temperature to 42
°C. The 5 incubators located under the microplates enable all test steps to be performed without moving
the microplate into specific areas.
Under the working area, NEXgen can be equipped with a relevant compartment - optional - that can
contain from 1 to up 4 positions with shakers and incubators. The shaking ranges from 200 to 1000 rpm.
NEXgen handles 5 different wash buffers plus 1 of distilled water.
The internal waste tank capacity is 8 liters of waste liquids.
The microplate is read by means of a state of the art LED reading system that is designed to guarantee high
performances, low maintenance, large measurement range and excellent reproducibility. The optics gives a
high resolution scan, 29 readings per each well and a fast reading, 8 seconds to read the whole microplate
at double wavelength.
A barc de reader identifies all resources loaded on the working area. The barcode reader is able to detect:
•The presence of vials, test-tubes, etc… in the positions requested by the software
•The barcode printed on the vials, test-tubes, etc…labels if it is present
The sensors on the working area guarantee the correct positioning of all racks and holders loaded on board.
OpenLAB is the NEXgen system software. It is based on essential graphics and all necessary information for
the operator to rapidly perform the loading, assay runs and results management procedures make the
NEXgen instrument an easy to use instrument.
1.7 E
NVIRONMENTAL PROTECTION INSTRUCTIONS
1.7.1 D
ISPOSAL PRECAUTIONS
NEXgen must be considered as a non household waste and its waste must be disposed of in conformity to
the end-user’s national laws on electric and electronics waste.

NEXgen User’s Manual
Introduction
11
NEXgen is built with materials that do not harm the environment; during its usage it can be contaminated
by infectious substances. It is recommended to decontaminate the NEXgen instrument before disposal
(refer to Waste disposal on page 31)
Make sure that NEXgen is disposed of in a proper way in order to avoid:
•potential environmental damages resulting from an improper disposal of the instrument
•administrative penalties resulting from an illegal waste disposal.
1.7.2 D
ECONTAMINATION
Decontamination of NEXgen must be carried out by wearing a lab coat in order to shelter your clothes from
contaminating agents and protect the skin from the contaminating agents exposure, disposable latex or
vinyl gloves, to prevent the direct contact with the potentially contaminated NEXgen parts and a facial
mask, to protect the mucosae, eyes, nose and mouth from any potential contaminating agents.
Prepare two solutions of sodium hypochlorite (commercial hypochlorite): one at 1% and the other at 0.5%.
The commercial sodium hypochlorite concentration is generally 5.25%; such solutions must be prepared as
follows:
Solution A (1%): 200 mL of sodium hypochlorite and 800 mL of deionized water;
Solution B (0.5%): 100 mL of sodium hypochlorite and 900 mL of deionized water.
WARNING
:
Plea
se contact the Manufacturer before decontaminating
NEXgen
through different
methods than the ones recommended in this chapter.
WARNING
:
The decontamination procedure proposed in this section does not guarantee the complete
sterilization of NEXgen or that all viruses and microorganisms are completely inactivated. In
spite of this, this procedure allows minimizing the contamination hazard.
W
ORKING AREA DECONTAMINATION
Remove all the resources from the working area (racks, holders, etc..).
Soak an absorbent non-fibrous towel in A Solution to clean the surfaces of the instrument. In order to get
an acceptable decontamination level, leave A Solution for 15 minutes at least, then remove the sodium
hypochlorite by using an absorbent cotton swab soaked in the deionized water.
R
ACK DECONTAMINATION
Remove the racks from their positions and remove the possible resources that must be disposed of in the
specially provided containers.
Soak an absorbent non fibrous towel with A Solution to clean the rack surfaces and holes. Leave A Solution
for 10 minutes at least, then remove the sodium hypochlorite by using an absorbent cotton wad soaked in
the deionized water.

NEXgen User’s Manual
Introduction
12
U
SED TIPS CASE DECONTAMINATION
Remove the used tips case from its position and make sure any left tips are removed from the inside;
dispose of the tips in the supplied special containers. Clean the case by using an absorbent towel soaked in
B solution. Leave the solution on for 10 minutes at least, then remove the solution by using an absorbent
towel soaked in the deionized water.
Place the used tips case back to its position.
M
ANIFOLD AND WASTE LIQUIDS TANK DECONTAMINATION
From the position number 6 of the tank drawer, remove the tank number 6 with red label and empty the
distilled water usually contained in it. Fill the same tank with 2 L of B Solution.
Empty the waste liquids tank as follows: from the main menu screen, click on end of work then click on start
empty waste. Wait for the tank to be completely emptied, then click on end empty waste.
To decontaminate the manifold and the waste liquids tank, click on end of work from the main menu
screen, then click on clean wash system and wait for the cycle to be completed. Repeat the procedure until
the emptying of tank number 6 is completed. At the end, clean tank number 6 with distilled water and fill it
with 2 L of distilled water and reposition it in the tanks drawer in number 6 position.
Empty the waste liquids tank containing B Solution by following the procedure described above.
1.8 P
ACKAGE CONTENTS
NEXgen basic accessories list
Code
Description
Quantity
30004372
NEXgen
v.2
1
30600980
Operator's Manual in English
1
30600990
Service Manual in English (for distributors)
1
30650090
Multilingual Operator's Manual CD
1
30213711
Samples rack
6
30213721
Reagents an
d controls rack
6
30213741
Disposable tips rack
1
30213750
Microplate holder
6
30213961
IFA slides rack
2
30214270
Dilution plates rack (5)
1
26603
200 µL Disposable tips
960/1
26606
1000 µL Disposable tips
960/1
62910
Dilution plates
1
10163341
Us
ed tips case
1
13000300
2L Wash buffers tank
6
20400500
10A Time
-
lag fuse 5x20mm UL
2
21890570
FTP CAT.5 RJ45 3mt Shielded cord
1

NEXgen User’s Manual
Introduction
13
Code
Description
Quantity
21890040
EU Power cord
1
21890370
USA Power cord
1
30214380
Valve drain pipe
1
30214390
External waste tank
1
30502560
NEXgen
transportation handles kit
1
19901070
Pipettors calibration tool
1
19901080
Manifold calibration tool
1
19901090
Aspiration needle cleaning tool
1
19901100
Dispensing needle cleaning tool
1
NEXgen optionals list
Code
Description
Quantity
302
14101
Shaker
-
incubator unit
4 max
30214150
Shakers/Heaters frame ass Y
Frame
30214160
Bottom heater unit
30214400
Bottom exchange unit
30213750
Microplate holder

NEXgen User’s Manual
General information
14
2. G
ENERAL INFORMATION
2.1 T
ECHNICAL SPECIFICATIONS
2.1.1 S
AMPLES RACK
Image 4 - Samples rack
NEXgen is supplied with 6 sample racks that can be removed and loaded on the instrument allowing them
to slide on the working area slots. The insertion of test-tubes with different diameters into the rack and
their support are facilitated by springs that ensure the stability. Each single rack is identified by a unique
barcode. Inside the rack, each position has a barcode providing the following information.
a) Test-tube absence - the barcode reader reads the position back barcode;
b) Test-tube presence - the barcode reader reads the barcode in the label on the test-tube;
c) Test-tube wr ng p siti ning - the barcode reader gives no reading.
Samples rack specifications
Capacity
24
/28
samples
Test
-
tubes sizes
75 to 85 mm height
12 to 16 mm diameter
Minimum sample volume
100 µL
Sample positive identification
Yes
NEXgen User’s Manual
General information
15
2.1.2 D
ISPOSABLES RACK
Image 5 - Diluti n plate rack
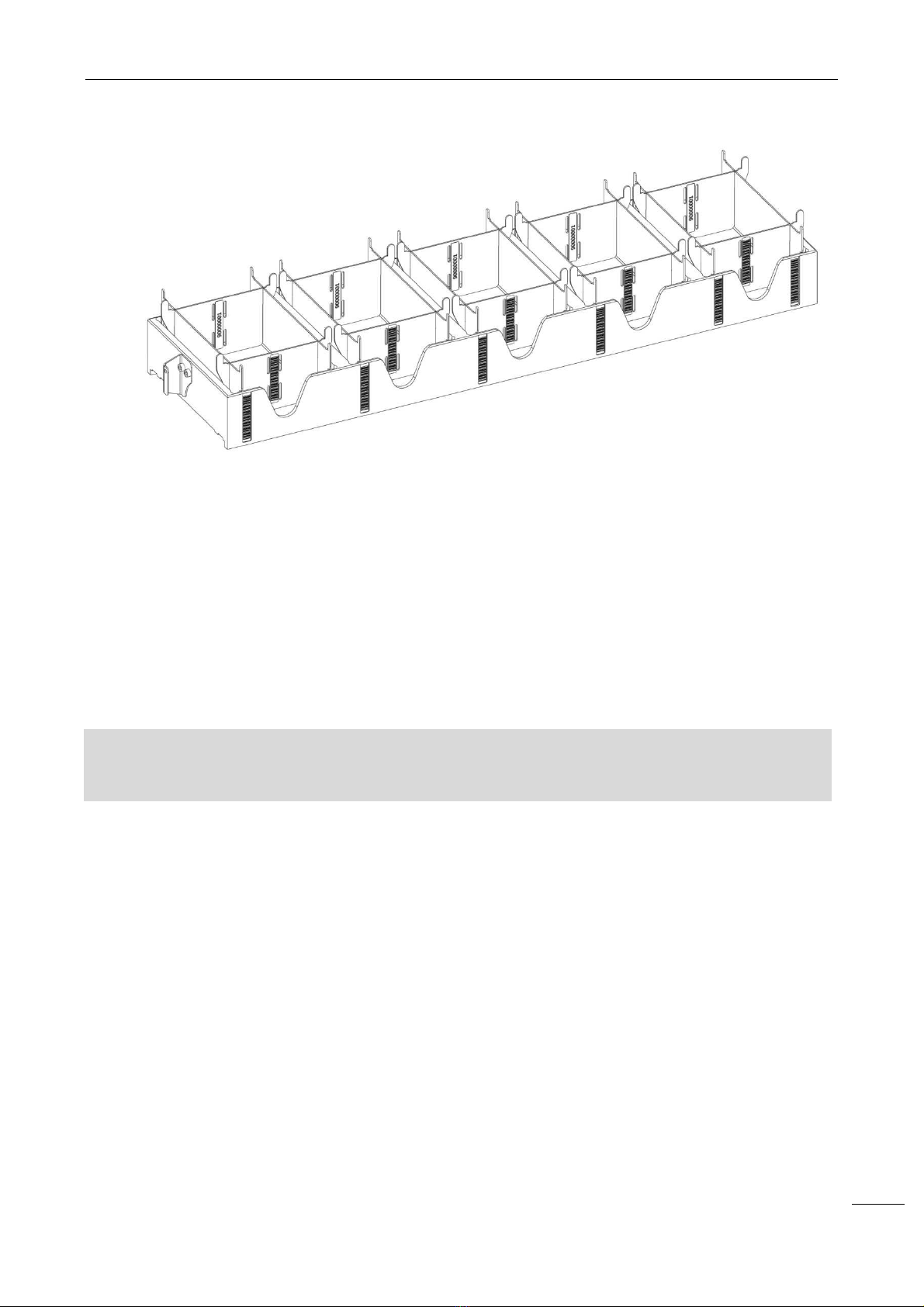
NEXgen is supplied with 2 rem vable racks where both dilution strips and disposable tips can be loaded.
Each rack consists of 5 positions; it can be loaded on the instrument by sliding it on the working area slots.
The racks are identified by a unique barcode. The standard instrument configuration comes with 3 holders
for the dilution strips and 8 for the disposable tips.
Each dilution strip consists of 8 wells, 2 mL each. Each support for the disposable tips contains 96 tips
(200µL or 1000µL).
Dilution strips and disposable tips must be purchased separately, please refer to NEXgen’ Sales
Department.
WARNING
:
After usa
ge, dilution
strips and disposable tips
have to be removed from the
holder
and
disposed of as contaminated biological material.
NEXgen User’s Manual
General information
16
Image 6 - Tips rack
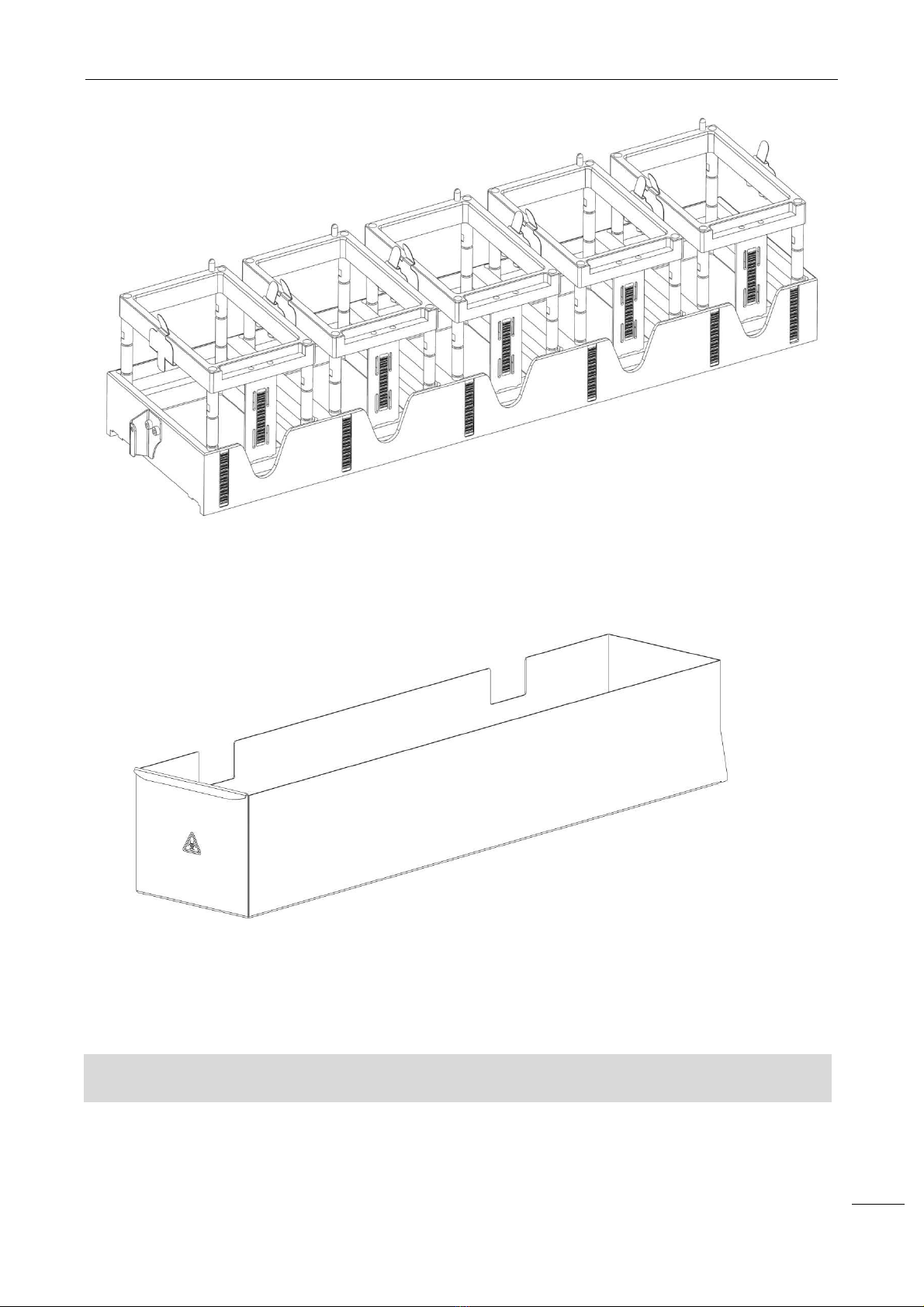
2.1.3 U
SED TIPS CASE
Image 7 - Used tips case
The used tips case is in a raised position on the working area, on the right side of NEXgen.
The software manages the release of the tips to happen randomly throughout the case length, avoiding the
tips to accumulate in a single area of the case.
WARNING
:
Used tips have t
o be handled and disposed of as contaminated biological material.

NEXgen User’s Manual
General information
17
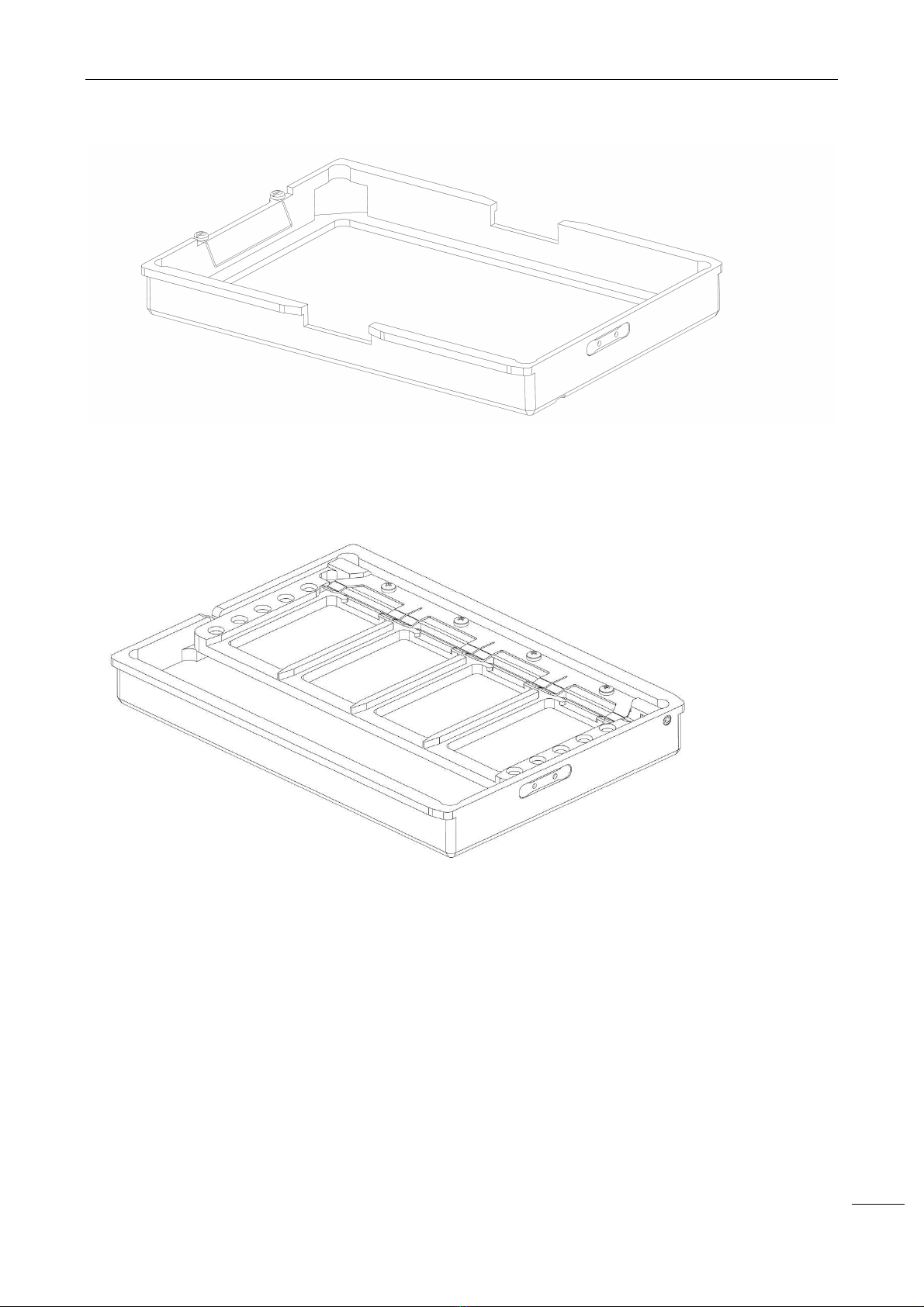
2.1.4 R
EAGENTS RACK
Image 8 - Reagents rack
NEXgen is supplied with 6 reagents racks that can be removed and loaded on the instrument allowing them
to slide on the working area slots. Each single rack is identified by a unique barcode. Inside the rack, each
position has a barcode that provides the following information:
a) reagent r c ntr l/calibrat r/standard vial absence - the barcode reader reads the position back
barcode;
b) reagent r c ntr l/calibrat r/standard vial presence - the barcode reader reads the barcode on
the vial;
c) reagent or control/calibrator/standard vial wrong positioning - the barcode reader gives no reading.
Reagents rack specifications
Capacity
12 positi
ons for standards and controls
5 positions for reagents
Reagents and controls/calibrators/
standards loading holes diameter
Customizable
Prediluted controls/calibrators/
standards minimum volume
50 µL
+ volume to be aspirated
Concentrated controls/calib
rators/
standards minimum volume
50 µL
+ volume to be aspirated
Reagents minimum volume in a 60 mL vial
4
000
µL
+ volume to be aspirated
Reagents minimum volume in a 15 mL vial
8
00
µL
+ volume to be aspirated
WARNING
:
The reagents vials have to be hand
led and disposed of as potentially contaminated
material.
NEXgen User’s Manual
General information
18
2.1.5 M
ICROPLATES HOLDER
Image 9 - Micr plate h lder
NEXgen is supplied with 6 micr plate h lders, each for a 96 well microplate.
2.1.6 IFA
SLIDES RACK
Image 10 - IFA slides rack
NEXgen is supplied with 2 IFA slide racks, 4 slides each. NEXgen can contain up to 7 IFA slide racks.

NEXgen User’s Manual
General information
19
2.1.7 R
EADING SYSTEM
Image 11 - Reading system
The reading system is located in the lower part of NEXgen. The microplate robotic arm moves the
microplate from the working area to the retractable cart of the reader.
Reading system specifications
Dynamic
0
-
3.
3
OD
Spectrum
400
-
700 nm
Loadable filters number
8
Precision
0,01
SD
(0,000
-
0,500
OD
)
≤1% CV (0,501-2,000 OD)
≤1,5% CV (2,001-2,500 OD)
≤2% CV (≥2,501 OD)
Linearity
< ± 0.75% (at 0.100 to 3.000 OD)
< ± 1.50% (at 3.000 to 3.300 OD)
Accuracy
< ± 0.50% (1.0 OD at 492 nm)
Reading time
5 seconds
per
single wavelength
8 seconds per dual wavelength

NEXgen User’s Manual
General information
20
2.1.8 B
ARCODE READER
Image 12 - Barc de reader
The barc de reader is on the external upright of the y-axis. Any resource loaded on NEXgen is identified by
the barcode reader.
Barcode reader specifications
Type
High Speed Laser Sca
n
Scanning mode
Single line
Scanning speed
500 scans/sec
Minimum code width
0.127 mm (5 mil)
Configured barcodes
EAN/UPC; Code 39; ITF 2 di 5; Code 93; Code 128
Interface
RS232C (baud rate 115200)
Table of contents
Popular Laboratory Equipment manuals by other brands

MLT
MLT FS-16-25 Instructions for use

Waters
Waters Xevo TQ MS Operator's, overview and maintenance guide

REPLIGEN
REPLIGEN TangenX PRO PD user guide

Ample Scientific
Ample Scientific Champion S-33 manual
Ecowell
Ecowell Homeguard manual

Molecular Devices
Molecular Devices FilterMax F3 Unpacking and setup guide