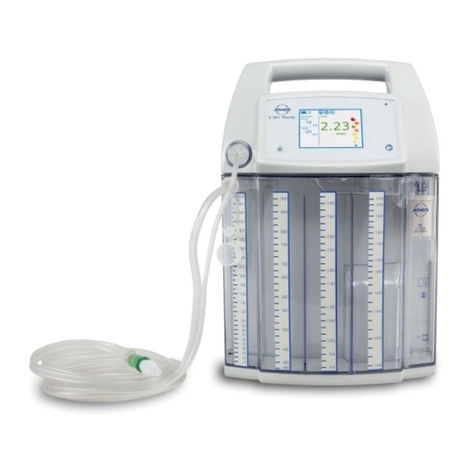
Atmos C 451 User manual

Operating Instructions
ATMOS C 451
English
GA1GB.210102.0
2022-02 Index 23 0124

2
Table of contents
1 Introduction.......................................................................................................4
1.1 Notes on operating instructions ..............................................................................4
1.2 Explanation of pictures and symbols ......................................................................6
1.3 Intended use...............................................................................................................8
1.4 Function.......................................................................................................................9
1.5 Intended users ...........................................................................................................9
1.6 Scope of delivery........................................................................................................9
1.7 Transport and storage............................................................................................ 11
2 Notices for your safety...................................................................................13
2.1 General safety instructions.................................................................................... 13
2.2 Danger for users, patients, and third parties ...................................................... 13
2.3 Avoiding damage to the device ............................................................................. 15
3 Setting up and starting up .............................................................................16
3.1 Device overview....................................................................................................... 16
4 Operation.........................................................................................................17
4.1 Initial start-up .......................................................................................................... 17
4.2 Preparing the device............................................................................................... 17
4.3 Assembly of the DDS secretion canister .............................................................. 17
4.4 Using the DDS splash protection .......................................................................... 17
4.5 Attaching/removing the DDS secretion canister lid............................................ 17
4.6 InsertingandremovingtheDDSbacterialandvirallter/oversuctionstop . 18
4.7 Attaching, closing, and opening the DDS secretion canister handle................ 18
4.8 Attaching/removing the DDS secretion canister ................................................. 18
4.9 DDS secretion canister hose holder ..................................................................... 18
4.10 Inserting the DDS hose adapter............................................................................ 19
4.11 Connecting the suction hose ................................................................................. 19
4.12 On/oswitch ........................................................................................................... 19
4.13 Adjusting the vacuum............................................................................................. 19
4.14 Suctioning ................................................................................................................ 20
4.15 CheckingtheDDSbacterialandvirallter/oversuctionstop.......................... 20
5 Options.............................................................................................................21
5.1 Securing the device................................................................................................. 22
5.2 Walking with the system trolley............................................................................. 22
5.3 DDS changeover docking station .......................................................................... 22
5.4 Tray for the system trolley base ............................................................................ 23
5.5 Potential equalisation............................................................................................. 23
5.6 Use of disposable suction systems (Serres®, Medi-Vac®, Receptal®) ................ 24
6 Reprocessing....................................................................................................25
6.1 Safety instructions for reprocessing..................................................................... 25
6.1.1 General safety instructions............................................................................... 25
6.1.2 Danger for users, patients, and third parties................................................. 25
6.1.3 Avoiding damage to the device........................................................................ 25
6.2 Preparing surfaces .................................................................................................. 26
6.2.1 Overview ............................................................................................................. 26
6.2.2 Selecting process chemicals............................................................................. 26
6.2.3 Pre-cleaning........................................................................................................ 27
6.2.4 Wipe disinfection ............................................................................................... 27

3
6.3 Reprocessing the accessories................................................................................ 27
6.3.1 Overview ............................................................................................................. 27
6.3.2 Selecting process chemicals............................................................................. 28
6.3.3 Secretion canister system................................................................................. 28
7 Maintenance and service...............................................................................30
7.1 Periodic tests ........................................................................................................... 30
7.2 Function check......................................................................................................... 30
7.3 Sending in the device.............................................................................................. 30
7.4 Reprocessing by the manufacturer....................................................................... 30
7.5 Changing the fuse ................................................................................................... 31
8 Troubleshooting ..............................................................................................32
9 Accessories ......................................................................................................33
10 Consumables ...................................................................................................35
11 Disposal............................................................................................................36
12 Technical data .................................................................................................37
13 Notes on EMC ..................................................................................................39
Further information, accessories, consumables
and spare parts are available from:
ATMOS
MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Str. 16
79853 Lenzkirch
Germany
Phone +49 7653 689-0
FAX +49 7653 689-190 (Switchboard)
FAX +49 7653 689-393 (Service)
E-mail: [email protected]
Web: www.atmosmed.de

4Introduction
1 Introduction
1.1 Notes on operating instructions
These operating instructions contain important information on how to
operateyourproductsafely,correctly,andeectively.
These operating instructions are designed for training and instructing
operating personnel in the use of the system, and they are also intend-
ed for use as a reference manual. This document may only be reprint-
ed, either in part or in whole, with written permission from ATMOS.
These operating instructions must always be kept available near
the product.
Care, periodic tests, regular cleaning, and proper use are essential.
They ensure the operational safety and usability of the product.
Maintenance, repairs, and periodic tests may only be carried out
by persons who have the appropriate technical knowledge and are
familiar with the product. The person in question must possess the
necessary test devices and original spare parts required to carry out
these measures.

5Introduction
Read chapter ‘Notices for your safety’ on page 13 before using the
productforthersttime.Thiswillhelpyoutoavoidpotentiallydanger-
ous situations.
The product bears the CE marking CE 0124 in accordance with the EC
Council Directive 93/42/EEC concerning medical devices and meets the
basic requirements of Annex I of this directive.
The product complies with all applicable requirements of Directive
2011/65/EU on the restriction of the use of certain hazardous sub-
stances in electrical and electronic equipment (‘RoHS’).
The Declarations of Conformity and our General Terms and Conditions
can be viewed on our website at www.atmosmed.com.
ThequalitymanagementsystematATMOShasbeencertiedaccord-
ing to the international standard EN ISO 13485.
These operating instructions are valid for the following devices:
• ATMOS C 451 340.0300.0
• ATMOSC451mobilewith2x1.5lcanisters–fullyassembled 340.0376.0
• ATMOSC451mobilewith2x1.5lcanisters–forself-assembly 340.0335.0
• ATMOSC451mobilewith2x3lcanisters–fullyassembled 340.0377.0
• ATMOSC451mobilewith2x3lcanisters–forself-assembly 340.0334.0
• ATMOSC451mobilewith2x5lcanisters–fullyassembled 340.0378.0
• ATMOSC451mobilewith2x5lcanisters–forself-assembly 340.0333.0
• ATMOSC451withpracticepackage1.5l 340.0339.0
• ATMOSC451withpracticepackage3l 340.0338.0
• ATMOSC451portablewithstandardrail 340.0357.0
• ATMOSC451mobilewithstandardrail–forself-assembly 340.0358.0
• ATMOSC451mobilewithstandardrail–fullyassembled 340.0375.0
1.2 Explanation of pictures and symbols
In the operating instructions

6Introduction
DANGER
Warning of a danger that will result in immediate fatal or serious injury. Observe the
necessary measures.
WARNING
Warning of a danger that can cause fatal or serious injury. Observe the necessary
measures.
CAUTION
Warning of a danger that can cause minor injury. Observe the necessary measures.
ATTENTION
Notice of a danger that can damage the product or other objects. Observe the neces-
sary measures.
Warning of a danger that can cause fatal or serious injury.
Notice of potential material damage.
Useful information on the handling of the device.
1. Action. Proceed step by step.
»Result of an action.
Move in this direction, plug in.
On device and type plate
Follow operating instructions (blue)
Observe the operating instructions
Warning; pay special attention
0124
This product complies with the relevant requirements of EU directives.
This product complies with the relevant requirements of EU directives.
Foot switch
Manufacturer
Manufacturing date
SN Serial number
REF Order number

7Introduction
EAN European Article Number
IPX1 Protection against the penetration of harmful moisture (dripping water)
Application part type BF
Professional disposal
For single use only (symbol located on consumables)
NON
STERILE
Not sterile
AUTOCLAVE
Autoclavable
PATIENT Connection for suction hose / patient
LATEX
No natural rubber latex
Potential equalisation
Protection class II
Fuse
Alternating current
Device on
Deviceo(standbyforoptionalfootswitchmode)
Fragile, handle with care
Store in a dry place
Protect from sunlight
GOSTCerticate(Russia)
Eurasian conformity

8Introduction
1.3 Intended use
Name: ATMOS C 451
Main function: Suctionofsecretions,blood,serousuids,rinsinguids,and
forthetemporarycollectionoftheseuids.
Medical indications/
application:
For all applications where suction is needed, such as in gener-
al surgical procedures (e.g., suction of wound cavities, ab-
scesses), suction of the nasopharyngeal cavity, in endoscopy
forsuctionofsecretionorrinsinguids,andinneurosurgery.
For vacuum extraction.
Specication of the
main function:
Drainageandtemporarycollectionofbodyuids.Anelectric
suction pump is used to generate negative pressure. An addi-
tional secretion canister must be attached to allow temporary
collectionofthedrainedbodyuids.
User prole: Doctors,medicalstawithoutrestrictions.
Patient group: Patients of all ages with and without restrictions.
Application organ: Naturaloricesandopeningsresultingfromasurgicalinter-
vention (entire body; human and animals).
Application time: For short-term use on patients (< 30 days)
Area of application: The application site is the clinical, outpatient, private practice,
andveterinaryeld.Thedevicemayonlybeusedbypersons
who have received the relevant training and instruction.
Contraindications: Not suitable for:
• Drainage operations in the low-vacuum range (e.g.,
thoracic or wound drainage)
• Use outside the medical areas
• Suctionofammable,corrosive,orexplosivesubstances
• Suction in potentially explosive atmospheres
• Suction of surgical smoke in conjunction with HF, electri-
cal, or laser surgery
The product is: active
Sterility: Product not sterile
Disposable product /
Reprocessing:
The device and part of the accessories are reusable. For infor-
mation on reprocessing, cleaning, and disinfection, please see
the operating instructions.

9Introduction
1.1 Function
• TheATMOSC451isamains-operatedsurgicalsuctiondevice,thecoreofwhichisa
high-performance, maintenance-free diaphragm pump. It generates vacuum in the
suction hose and secretion canister system, which assists in suctioning and collect-
ingsecretions.Viaavacuumregulatorwithvacuumgauge,thenalvacuumand
thus the desired suction capacity can be precisely adjusted.
• Secretioncanistersofdierentsizesareavailableforsecretioncollection.The
reusablesecretioncanisterscanbeattachedtotheATMOSC451viatheDirect
Docking System. The user can connect the suction hose directly. A hydrophobic DDS
bacterialandvirallterlocatedinthecanisterlidpreventsbacteria,virusesand
liquidsfromenteringthedevice.Thislterprotectsthedeviceagainstoversuction.
The intake located in the hose connection prevents foaming in the secretion canister
andthereforeensuresalongerlterlife.
• A system trolley with extensive accessories is available for mobile use.
1.4 Intended users
May only be used by trained professionals in supervised and medical operations.
1.5 Scope of delivery
Name
340.0300.0
ATMOS C 451
Basic device
Power cable 5 m 008.0818.0
Operating Instructions
Name REF
340.0376.0/340.0335.0
ATMOSC451 mobile with 2 x 1.5 l canisters
1xATMOSC451 340.0300.0
2x DDS secretion canister, plastic, 1.5 l, autoclavable 340.0050.0
2x DDS canister lid with sealings, autoclavable 340.0053.0
2x DDS canister handle, blue, autoclavable 340.0326.0
2x DDS splash protection, silicone, autoclavable 340.0056.0
1x DDS hose adapter set (Ø 6 mm + Ø 10 mm), autoclavable 340.0057.0
1x hose holder, stainless steel 320.0611.0
1x standard rail adapter 340.0331.0
1xsystemtrolleyATMOSC451withDDSchangeoverstation 340.0171.0
10xhydrophobicDDSbacterialandvirallter 340.0054.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0

10 Introduction
Name REF
340.0377.0/340.0334.0
ATMOSC451 mobile with 2 x 3l canisters
1xATMOSC451 340.0300.0
2x DDS secretion canister, plastic, 3 l, autoclavable 340.0051.0
2x DDS canister lid with sealings, autoclavable 340.0053.0
2x DDS canister handle, blue, autoclavable 340.0326.0
2x DDS splash protection, silicone, autoclavable 340.0056.0
1x DDS hose adapter set (Ø 6 mm + Ø 10 mm), autoclavable 340.0057.0
1x hose holder, stainless steel 320.0611.0
1x standard rail adapter 340.0331.0
1xsystemtrolleyATMOSC451withDDSchangeoverstation 340.0171.0
10xhydrophobicDDSbacterialandvirallter 340.0054.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0
Name REF
340.0378.0/340.0333.0
ATMOSC451 mobile with 2 x 5l canisters
1xATMOSC451 340.0300.0
2x DDS secretion canister, plastic, 5 l, autoclavable 340.0052.0
2x DDS canister lid with sealings, autoclavable 340.0053.0
2x DDS canister handle, blue, autoclavable 340.0326.0
2x DDS splash protection, silicone, autoclavable 340.0056.0
1x DDS hose adapter set (Ø 6 mm + Ø 10 mm), autoclavable 340.0057.0
1x hose holder, stainless steel 320.0611.0
1x standard rail adapter 340.0331.0
1xsystemtrolleyATMOSC451withDDSchangeoverstation 340.0171.0
10xhydrophobicDDSbacterialandvirallter 340.0054.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0
Name REF
340.0339.0
ATMOSC451 with practice package 1.5 l
1xATMOSC451 340.0300.0
1x DDS secretion canister, plastic, 1.5 l, autoclavable 340.0050.0
1x DDS canister lid with sealings, autoclavable 340.0053.0
1x DDS canister handle, blue, autoclavable 340.0326.0
1x DDS splash protection, silicone, autoclavable 340.0056.0
1x DDS hose adapter set (Ø 6 mm + Ø 10 mm), autoclavable 340.0057.0
1x canister hose holder, autoclavable 340.0066.0
10xhydrophobicDDSbacterialandvirallter 340.0054.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0

11Introduction
Name REF
340.0338.0
ATMOSC451 with practice package 3l
1xATMOSC451 340.0300.0
1x DDS secretion canister, plastic, 3 l, autoclavable 340.0051.0
1x DDS canister lid with sealings, autoclavable 340.0053.0
1x DDS canister handle, blue, autoclavable 340.0326.0
1x DDS splash protection, silicone, autoclavable 340.0056.0
1x DDS hose adapter set (Ø 6 mm + Ø 10 mm), autoclavable 340.0057.0
1x canister hose holder, autoclavable 340.0066.0
10xhydrophobicDDSbacterialandvirallter 340.0054.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0
Name REF
340.0357.0
ATMOSC451 portable with standard rail
1xATMOSC451 340.0300.0
1x standard rail adapter 340.0330.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0
Name REF
340.0358.0/340.0375.0
ATMOSC451 mobile with standard rail
1xATMOSC451 340.0300.0
1xsystemtrolleyATMOSC451 340.0171.0
1x standard rail set for system trolley 340.0081.0
1x hose for the connection of the device and canister system 340.0083.0
1x hose holder, stainless steel 320.0611.0
1x standard rail adapter 340.0331.0
1x suction hose (silicone), Ø 6 mm, L = 2 m 000.0361.0
1.6 Transport and storage
Onlytransporttheproductinashippingcartonthatispaddedandoerssucient
protection.
If damage occurs during transport:
1. Document and report damages in transit.
2. Send the device to ATMOS; see chapter ‘7.3 Sending in the device’ on page 30.
Ambient conditions for transport and storage:
• Temperature: −30...+50°C
• Relative humidity: 5...90% without condensation
• Air pressure: 700...1060hPa

12 Introduction
Storage:
Aftertransportingthedeviceattemperaturesbelow0°C,keepitatroomtempera-
ture for at least six hours before initial start-up. If the device has not been acclima-
tised, it may not be operated as the electronic components may become damaged.

13Notices for your safety
2 Notices for your safety
2.1 General safety instructions
The product only meets the safety requirements of users, patients, and third parties
when it is fully functional. Therefore, observe the following instructions on your product:
Please read and pay attention to the safety instructions prior to using the product.
2.2 Danger for users, patients, and third parties
WARNING
Choking hazard for children due to accessories!
Children can strangle themselves or choke on small parts.
• Keep children away from hoses and connection cables.
• Keep children away from swallowable small parts. Examples of swallowable small
partsarethengertipandsealingring.
CAUTION
Explosion and re hazard!
Burns and injuries are possible.
• Neversuctionanyexplosive,ammable,orcorrosivegasesorliquids.Pleaseob-
serve the product’s intended use in chapter ‘Intended use’ on page 8.
• Never operate the product in potentially explosive areas or areas that are oxygenat-
ed.
• Only use original accessories and original spare parts from ATMOS. This applies to
the power cable in particular.
WARNING
Avoid misuse.
Your patient can be severely injured.
• The product may only be used by medically trained persons who have been instruct-
ed in the handling of the medical suction system.
• Theproductmayonlybeusedbyqualiedpersonnelinsupervisedoperation.
• Select the vacuum according to the patient and the application.
• Observe the valid guidelines.
• Always set up the device in such a way that the operating elements are in clear view
and within easy reach of the operator. The device must be placed on a stable, level
surface.

14 Notices for your safety
WARNING
Ensure that the device is always functional and ready for use.
Yourpatientcouldsuocate.
• Before connecting the device, check whether the required mains voltage on the
device matches the mains voltage of the mains power supply.
• Position the device in an easily accessible location and keep access free.
• Make sure that the power cable is working. Replace defective accessories immedi-
ately.
• Always carry out a function check before using the device.
• ATMOS recommends always having another suction device ready at hand. That way
you can also perform suctioning if a device should fail.
WARNING
Risk of infection due to pathogens on the product!
Deadly diseases may be transmitted.
• Always wear disposable gloves if you might come into contact with secretion.
• Always wear disposable gloves when using the product.
• Never use components that are marked with more than once. These components
are intended for single use only.
• Sterile-packed parts may only be used if the packaging is undamaged.
• Neveroperatethedevicewithoutabacterialandvirallter.
• A suction catheter, hose-rinsing aperture, or medical suction set must always be
connected to the hose. The suction hose must never come into direct contact with
the suction area.
• Clean and disinfect the product after every use.
• Clean and disinfect the product according to the operating instructions.
• The product must not be used following oversuction.
WARNING
Tripping hazard due to cables.
Injuries and fractures are possible.
• Lay the power cable properly.

15Notices for your safety
WARNING
Electric shock due to unsuitable power connection, incorrect handling of the
product, or damaged product components.
Burns, cardiac arrhythmias, and even fatal injury are possible.
• Do not operate the device if it has been dropped. In this case, clean and disinfect the
device and send it to ATMOS for repair.
• Always check the device and power cable for damage before every use. Do not oper-
ate the device if you notice any damage. In this case, clean and disinfect the device
and send it to ATMOS for repair.
• Disconnect the device from the mains power supply prior to cleaning or disinfection.
• You an only disconnect the device by removing the power plug from the mains
power supply.
• Position the device in such a way that you can easily disconnect it from the mains
power supply at any time.
• Never connect the device to a mains power supply without a protective conductor.
• Never touch the power cable with wet hands.
• Use the power cable only in a dry environment. The surroundings must be non-con-
ductive.
• Ensure that no liquid penetrates the device. If liquid has entered the device, op-
eration of the device must cease immediately. In this case, clean and disinfect the
device and send it to ATMOS for repair.
• Use the power cable only according to the operating instructions.
• Only use proper mains connections and extension cords.
• Never touch the device's interfaces and the patient at the same time!
• Only use original accessories and original spare parts from ATMOS. This applies to
the power cable in particular.
• Observethespecicationsregardingperiodictestsinchapter‘7.1Periodictests’on
page 30.
• Assembly, new settings, alterations, extensions, and repairs may only be carried out
by authorised persons.
• Do not modify the device without the manufacturer’s permission.
2.3 Avoiding damage to the device
ATTENTION
Storage and operation in an unsuitable environment.
The product may become damaged.
• Please observe the ambient conditions regarding transport, storage, and operation.
• After transporting the device at low temperatures, keep it at room temperature for
at least six hours before initial start-up. If the device is not acclimatised, it may not
be used as the diaphragms of the pump may become damaged.

16 Setting up and starting up
3 Setting up and starting up
Alwaysplacethedeviceonarmandlevelsurface.
3.1 Device overview
Front view
DDS secretion canister lid
DDS secretion canister
Vacuum gauge
On/oswitch
Vacuum regulator
Vacuum connection: Direct Docking System
The vacuum connection between the pump and
secretion canister is established immediately
when the DDS secretion canister is attached.
Connector panel on the bottom of the device
Connecting the power cable:
Only use power cables with angled IEC connec-
tors!
• Check that the mains voltage and mains frequen-
cyspeciedonthedevicecorrespondtothe
values of the mains power supply.
Connecting the foot switch (option):
• Slide the knurled nut onto the hose of the foot
switch.
• Insert the hose of the foot switch onto the con-
necting nipple.
• Tighten the knurled nut
Vacuum connection on the bottom for the system
trolley
• Toconnect,pressthecouplerrmlyintothe
jack until it locks into place (serves to establish
a vacuum connection of the secretion canisters
attached to the system trolley).
• To remove, press the metal release button on the
side and pull the hose out of the jack.

17Operation
4 Operation
4.1 Initial start-up
Observe the safety instructions prior to initial start-up!
After transporting the device at low temperatures, it should be kept at room temper-
ature for at least six hours before initial start-up; otherwise, the device may not be
operated.
4.2 Preparing the device
• Checkthatthevoltageandfrequencyinformationspeciedonthedevicecorre-
sponds to the values of the mains power supply and then connect the device to the
mains.
» The device is now ready for use.
4.3 Assembly of the DDS secretion canister
DDS secretion canister handle
HydrophobicDDSbacterialandvirallter
DDS hose adapter
DDS canister lid
DDS splash protection
DDS secretion canister
4.4 Using the DDS splash protection
1. Insert the splash protection to the connection on
the DDS canister lid.
The splash protection protects the DDS bacterial
andvirallteragainstprematuremoisteningby
liquids and/or foam generation.
4.5 Attaching/removing the DDS secretion canister lid
1. PlacetheDDSsecretioncanisteronarmsurfaceandset the DDS secretion canis-
ter lid horizontally on it (the lid cannot be turned incorrectly).
2. Gently press the DDS secretion canister lid with both hands onto the DDS secretion
canister as far as it will go.
3. To open the DDS canister lid, hold it on the reinforcement bars of the mounting
xtureandthenpulltheDDScanisterlidupandobyreachingintotheopeningfor
thelter.

18 Operation
4.6 Inserting and removing the DDS bacterial and viral
lter / oversuction stop
TheDDSbacterialandvirallter/oversuctionstopis
intended for single use only.
Before each use, check that the DDS bacterial and
virallter/oversuctionstopisdryandclean.Re-
placetheDDSbacterialandvirallterwithanew
DDSbacterialandvirallterifitisdiscolouredor
contaminated or if oversuction has occurred.
• InserttheDDSbacterialandvirallterontothe
DDS secretion canister handle.
4.7 Attaching, closing, and opening the DDS secretion
canister handle
1. To attach the DDS secretion canister handle,
insert it into the grooves of the canister lid (with
the locking latches open).
2. To close the DDS secretion canister handle, clip
the locking latches under the canister rim. Then
press the clips towards the secretion canister
until they click into place.
3. To open, pull the clips outwards and remove the
locking latches from under the canister rim.
4.8 Attaching/removing the DDS secretion canister
1. To attach the DDS secretion canister, allow it to
slide vertically downwards into the mounting
xture.
2. To remove the DDS secretion canister, lift it
vertically upwards.
4.9 DDS secretion canister hose holder
1. If using a DDS secretion canister hose holder,
attach it between the canister lid and the hose
adapter.
4.10 Inserting the DDS hose adapter
1. Insert the DDS hose adapter (Ø 6 or 10 mm) into
the ‘Patient’ opening on the DDS canister lid.
2. Turn it slightly and press it down.
The adapter can be removed again by turning it
slightly.

19Operation
4.11 Connecting the suction hose
Connect the suction hose to the already inserted
hose adapter.
4.12 On/o switch
• Press the “I” symbol to switch on the device.
• Pressthe“O”symboltoswitchothedevice.
• In position ‘O’ the device is ready for optional foot
switch operation (see options).
4.13 Adjusting the vacuum
• Close the suction hose and set the desired vacu-
um by turning the vacuum regulator according to
the direction of the arrow.
Please do not use brute force!
• Test the system for leaks if the desired vacuum is
not achieved.
4.14 Suctioning
1. Please ensure that the following parts have been reprocessed before treating a new
patient:
• Suction hose including hose-rinsing aperture or suction instruments
• Secretion canister including secretion canister lid and double hose connector
• Vacuum hose
2. Priortoeachuse,checkwhethertheDDSbacterialandvirallterwasinserted
during cleaning or disinfection.

20 Operation
3. ReplacetheDDSbacterialandvirallterwithanewDDSbacterialandvirallterifit
is discoloured or contaminated or if oversuction has occurred.
4. Switch on the device.
5. Close the suction hose and set the desired vacuum.
6. Connect the suction catheter, hose-rinsing aperture, or suction instruments.
Observe the liquid level in the secretion canister while suctioning. The DDS bacterial
andvirallterpreventsliquidfrombeingsuckedintothepump.Nevertheless,the
canister should be emptied or replaced when it is 2/3 full (including foam crown).
IfliquidhasbeensuckedintothepumpdespiteoftheDDSbacterialandvirallter,
the device may not be operated again until it has been checked by a authorized
service partner
4.15 Checking the DDS bacterial and viral lter /
oversuction stop
• TheDDSbacterialandvirallter/oversuctionstopisintendedforsingleuseonly.
Beforeeachuse,checkthattheDDSbacterialandvirallter/oversuctionstopisdry
andclean.ReplacetheDDSbacterialandvirallterwithanewoneifitisdiscoloured
or contaminated, or if oversuction has occurred.
ReplacetheDDSbacterialandviralltereverytimeyoucleanordisinfecttheDDS
canister system.
NeveroperatethedevicewithoutaDDSbacterialandvirallter/oversuctionstop.
Other manuals for C 451
2
Table of contents
Other Atmos Medical Equipment manuals

Atmos
Atmos S61 Servant User manual

Atmos
Atmos LS 21 LED User manual

Atmos
Atmos E 201 Thorax User manual

Atmos
Atmos Record 55 DDS User manual

Atmos
Atmos C 451 User manual

Atmos
Atmos Chair 21 P User manual

Atmos
Atmos A 161 Reference manual

Atmos
Atmos i View 21 User manual
Atmos
Atmos S 41 Gyne User manual

Atmos
Atmos C 11 User manual

Atmos
Atmos S 351 User manual

Atmos
Atmos Strobo 21 LED User manual
Atmos
Atmos S 201 Thorax User manual

Atmos
Atmos Variotherm plus User manual

Atmos
Atmos i View COLPO User manual

Atmos
Atmos Scope User manual
Atmos
Atmos C 051 Thorax User manual
Atmos
Atmos C 051 Thorax User manual

Atmos
Atmos iQam User manual

Atmos
Atmos ENT User manual