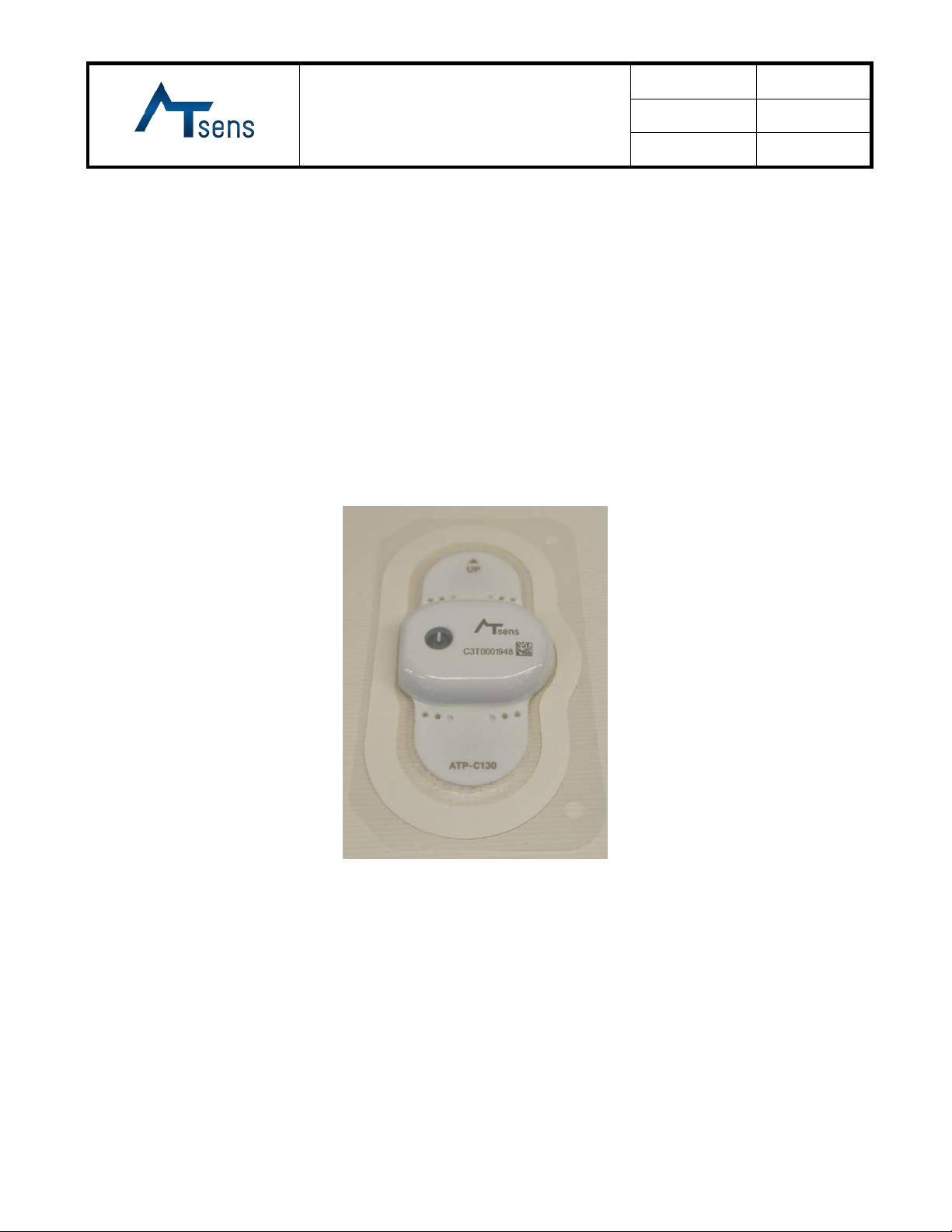
ATsens ATP-C130 User manual
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 1/ 123
User Manual
AT-PATCH ECG Analysis System
MODELATP-C130
ATsens Co.,Ltd.

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 2/ 123
※Revision History
No
Version
Revision Date
Revision Details
1
0.0
2020.10.30
Initial release
UM-C-002-User Manual_ATP-C130_Ver 0.0 _ 2020/10/30
Disposable Medical Device (Do Not Reuse) “This product is a medical device.”
The warranty period of this product is 12 months from the date of manufacture.
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 3/ 123
1. Product Introduction --------------------------------------------------------------------------------------------5
1.1 Intended Purpose
1.2 Packaging Information
2. Caution -------------------------------------------------------------------------------------------------------------7
2.1 Contraindications
2.2 Precautions
2.3 Device and App
2.4 S/W
2.5 Usage and Storage Conditions
2.6 Warning
3. Symbol Guide -----------------------------------------------------------------------------------------------------9
4. Device description ----------------------------------------------------------------------------------------------10
4.1 Operation principles
4.2 Operating system diagram
4.3 Device and accessories
5. Critical Component List --------------------------------------------------------------------------------------14
6. Patient Contact Part, Sterilization and Reusable --------------------------------------------------------14
7. Photograph and/or drawing of the device -----------------------------------------------------------------16
7.1 External Shape
7.2 Interface description
8. Insulation --------------------------------------------------------------------------------------------------------18
9. How to use (Device, App & PC S/W) -----------------------------------------------------------------------19
9.1 App (ATN-C30) Icon Screen and Initial launch Screen
9.2 Device Connection Window
9.3 Main Screen
9.4 LIVE Display Screen
9.5 Record Screen
9.6 Screens for Registering Symptom Note
9.7 Screens for Detailed View of Symptom Notes
9.8 View Details of Symptom Notes registered with the Device (ATP-C130)
9.9 Screen to Correct Symptom Notes
9.10 Screen to Search Recorded Data
9.11 Screen for 5 consecutive login failures
9.12 Screen when running theApp after along period of inactivity or 5 consecutive login failure
10. How to Use S/W (ATR-C130AT-Report PC S/W) ------------------------------------------------------33
10.1 Overview of AT-Report S/W
10.2 Installation of AT-Report PC S/W (ATR-C130)
10.3 Uninstalling AT-Report S/W ATR-C130
10.4 How to obtain S/W License
10.5 AT-Report (ATR-C130) PC S/W Title Bar
10.6 AT-Report (ATR-C130) Tab
10.7 Explanation of various operations of AT-Report
10.8 AT-Report Hotkey
10.9 Explanation of mouse operation in AT-report program
10.10 Displayed error message in AT-Report ATR-C30
11. How to Use the Device (ATP-C130) ----------------------------------------------------------------------111
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 4/ 123
11.1 How to Use and Attach the Device (ATP-C130)
12. How to Install theApp (ATN-C130) ---------------------------------------------------------------------114
13. Specifications -------------------------------------------------------------------------------------------------116
14. Usage and Storage Condition -----------------------------------------------------------------------------116
14.1 Conditions of Use
14.2 Storage conditions
15. Disposal --------------------------------------------------------------------------------------------------------117
16. Maintenance --------------------------------------------------------------------------------------------------117
16.1 Replacement of dedicated cable
17. Lifetime of the product & Block diagram --------------------------------------------------------------117
17.1 Lifetime of the product
17.2 Block diagram
18. Label -----------------------------------------------------------------------------------------------------------119
19. ANNEX1 –EMC (Electromagnetic Compatibility) Information ----------------------------------120
20. FAQs -----------------------------------------------------------------------------------------------------------123
Contact Us -------------------------------------------------------------------------------------------------------- 124

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 5/ 123
1. Product Introduction
It is a device that attaches electrodes to certain parts of the potential difference that occurs when myocardial
activity is transmitted to the body surface, which detects signals and uses radio signals to display this ECG
data. If you connect the App after turning on the power of the device, you can see the ECG signal through
the App. While device is running for 14 days, ECG records are stored in the device.After the use of the
device is completed for14 days, the record in the device is transmitted to the PC software through a
dedicated cable, and the final report is documented and printed through the analysis program of the PC
software.
1) Common Name: AT-Patch ECG Analysis System
2) Product Name (Model Name): AT-Patch ECG Analysis System (ATP-C130)
3) Manufacturer: ATsens Co.,Ltd.
4) Address: No.806, Point Town, 11 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, Korea
5) Contact: Tel. +82-70-5220-0738 / Fax. +82-70-8270-0738
6) U.S Representative
A. Name: Mtech Group
B. Address: 7707 Fannin St. Ste 200 Houston, TX 77054
C. The person in charge: Dave Kim
D. E-mail: davekim@mtech-inc.net
1.1 Intended Purpose
This device is intended to measure, analyze, and report continuous electrocardiogram (ECG) information for
long-term monitoring (up to 14 days) by attaching to the skin surface. While continuously recording patient
ECG, ECG records are saved in the device. It is indicated for use on patients 18 years or older who may be
asymptomatic or who may suffer from transient symptoms such as palpitations, shortness of breath,
dizziness, light-headedness, fatigue, or anxiety. The final report is offered to clinicians or doctors on an
advisory basis only. Used by patients as prescribed by physician or medical personnel.
1.1.1 Target Treatment group
Patients 18 years or older who may be asymptomatic or who may suffer from transient symptoms such as
palpitations, shortness of breath, dizziness, light-headedness, fatigue, or anxiety.
1.1.2 Target User
physician or qualified medical personnel (ex. a doctor, clinical pathologist, etc.)
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 6/ 123
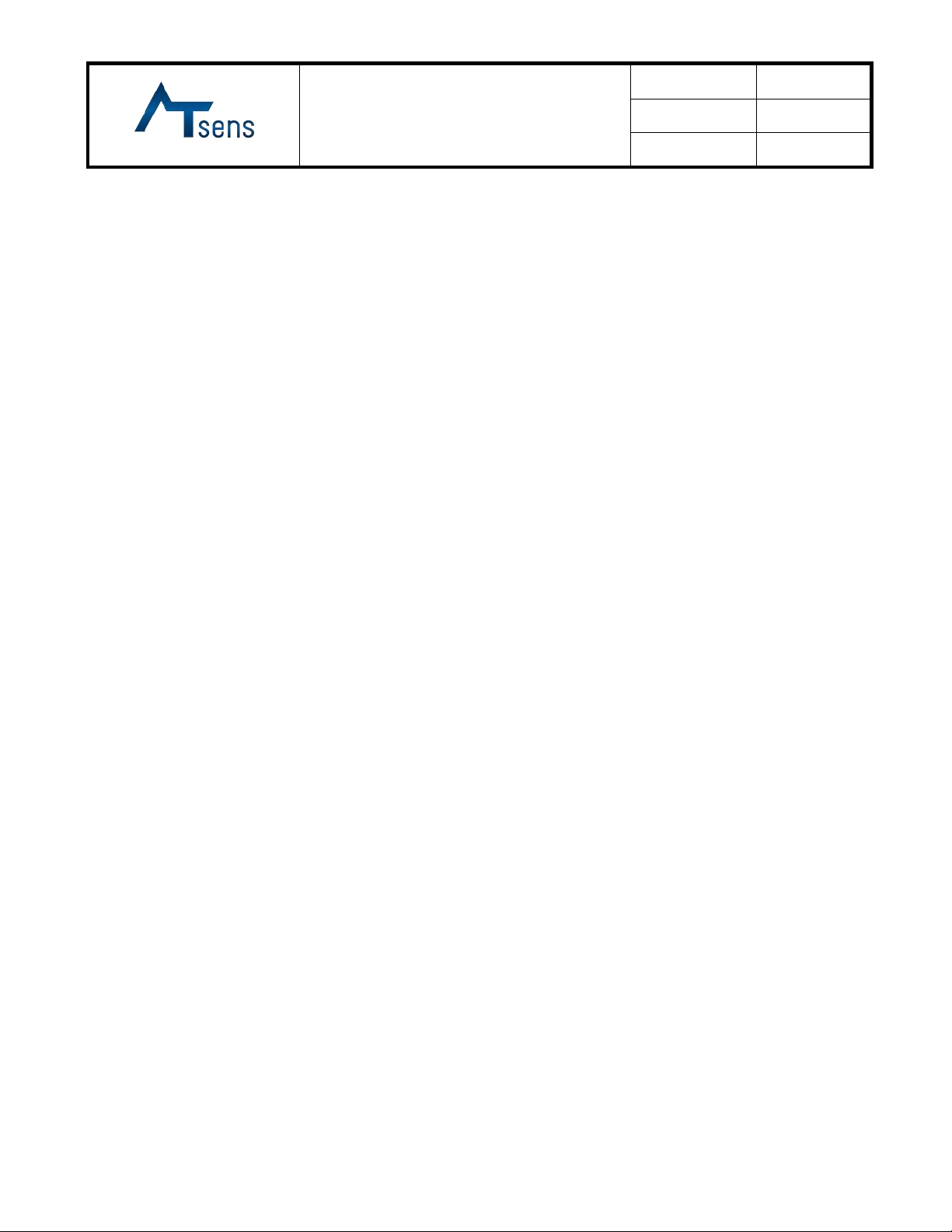
1.2 Packaging Information
1.2.1 Components (Device)
1) Basic
2) Accessories & PC S/W(ATR-C130)
Refer to User Manual 4.3.2
1.2.2 App
Android market: Google play (https://play.google.com/store)
iOS market: App Store(https://www.apple.com/ios/app-store)
1) Search for ‘AT note, ATsens, ATreport’in the Google Play™ Store or App StoreSM
2) Download app
3) Register before entering symptoms
* Supported onAndroid 5.0+ or iOS 12.0+
* See User manual 12 How to Install the App (ATN-C130) for details.
1.2.3 PC SW
Provide by USB memory: greater than or equal to 4GB & CE mark
Recommended System Requirements (PC)
Feature
Specification
Processor
Intel Core i7-9700K
RAM
16 gigabyte (GB)
Hard disk space
Main SSD: 512GB/Back-up HDD: 1TB
Graphics card
DirectX 9 or later with WDDM 1.0 driver
Display
1920 x 1080/24 inch Full-HD Monitor
OS
Windows 10 (64bit)

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 7/ 123
2. Caution
ATP-C130 is intended for direct use by the patient and the patiens is an intended operator.
2.1 Contraindications
1) If there are current symptoms or history of skin cancer, rash, skin disease, keloid, or injury, do not use our
product.
2) Do not use our product for patients with symptomatic events where instance variations in cardiac
performance could result in immediate danger to the patient.
3) Do not use our product in combination with cardiac defibrillators or high frequency surgical device near
strong magnetic fields such as MRI.
4) Do not use our product on patients who do not have the competency to wear the device for the prescribed
monitoring period.
2.2 Precautions
1) This device is a disposable product and cannot be reused. Reuse may lead to malfunctions or inaccurate
results.
2) Do not attach this product to any place other than the body application area.
3) Only authorized technicians are allowed to repair or disassemble this product.
4) Learn how to use this product through sufficient training before using this product.
5) Avoid using in places where there may be a problem with the wireless communication connection (where
there are many hardware and electronic devices).
6) Not available with defibrillator.
7) No exposure to strong electromagnetic fields.
8) Disposal
※When disposing of this product and battery, the waste disposal regulations in each region should be
followed. If the waste disposal regulations are not followed, it may cause environmental pollution. However,
the data of the product must be disposed of after processing.
2.3 Device and App
2.3.1 Cautions
1) Incorrect application and use of the sensor may lead to incorrect measurement, so avoid the following:
- Excessive patient movement
- Applications other than suitable body parts
- In order to prevent abnormalities in the signal according to the patient's skin condition, get sufficient
usage notice from a specialist before use.
2) The following people should consult the doctor before using the device.
- Sensitive or allergic skin patients
- If there is a wound on the skin coming into contact with the device
- Patients with cardiac pacemakers, cardiac defibrillators, or other implantable electrical devices.
- Pregnant women, breastfeeding mothers, infants, or children.
3) Be careful not to let any liquid enter the device. (Rating for water and dust resistance: IP 57)
4) Do not come into contact with organic compounds such as thinner or benzene.
5) Beware of strong shocks and vibrations.
6) Once you have attached the device to your body, do not reattach it.
7) In event of an unexpected operation or event, please record it inAT-note or contact distributor.
8)Some people may experience itching symptoms due to product attachment.
9) Do not disassemble the product
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 8/ 123
10) If the patch appears to be floating or falling off, apply medical tape to prevent the patch from falling off.
11) When connecting multiple BLEs (more than 2), the App does not work properly. Please stop using BLE
as much as possible while using the AT-note App.
12) When take a shower, be careful not to get soap, etc. on the patch as much as possible.
13) Federal (USA) law restricts the sale of this device to or on the order of a physician.
14) If you delete an app while using the device, you can reinstall the app, but the record for AT-note is
deleted. It is recommended not to delete the app while in use, except for forgotten password.
15) Adhere extra patches on the top of the attached patch when the previous patch is about to fall off.
2.4 S/W
2.4.1This Product is an auxiliary device to assist in the diagnosis. It may only be used to the extent that it. This
Product and installed equipment shall be capable of Bluetooth connection.
2.4.2 Cautions in Handling Patient Personal Information
2.4.3 It is very important to protect patient's personal information and comply with [Personal Information
Protection Act].
2.4.4 The patient's personal information will not be used for purposes other than the analysis and monitoring
of the patient's heart rhythm. If the purpose of use is changed, prior consent will be obtained.
1) The collected patient's personal information is stored and managed for up to 5 years.
2) When personal information becomes unnecessary due to the lapse of retention period or achievement of
processing purposes, the personal information shall be destroyed without delay.
3) The following measures are taken to ensure information safety.
- Administrative measures: Training on Personal Information Protection Act every year
- Technical measures: Encrypting personal information, installing security program, managing access to
personal information data and keeping access records for more than 6 months
- Physical measures: No access for unauthorized persons and the use of document encryption devices
2.5 Usage and Storage Conditions
2.5.1 Conditions of Use
1) Temperature range: 10-45℃
2) Range of relative humidity: 10-95%, non-condensing
3) Range of atmospheric pressure: 700-1060hPa
4) Rating for water and dust resistance: IP57
※The IP 57 rating prevents dust-proof dust from entering the interior. Even a slight intrusion of dust does
not impede normal operation. Protection against underwater immersion is not adversely affected by
immersion in water under the specified pressure and time.
2.5.2 Storage Conditions
1) Temperature range: -20-55℃
2) Range of relative humidity: 10-95%, non-condensing
3) Range of atmospheric pressure: 700-1060hPa
2.6 Warning
1) Do not use ATP-C130 on patients with known allergic reaction to adhesives or hydrogels or with family
history of adhesive skin allergies. Patient may experience skin irritation.
2) Do not reuse ATP-C130. This is a single use medical device. Reuse may cause incorrect patient data and
patient may experience skin irritation.
3) Do not useATP-C130 on patients residing in areas with limited to no smartphone reception.
4) If skin irritation such as severe itching or allergic symptoms develop, please remove the patch.

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 9/ 123
3. Symbol Guide
No.
Symbol
Descriptions
1
The serial number that identifies the object
2
Date of manufacture
3
For EuropeanAuthorised Representative
4
Keep dry
5
Prevents dust-proof dust from entering the interior. Even a slight intrusion
of dust does not impede normal operation. Protection against underwater
immersion is not adversely affected by immersion in water under the
specified pressure and time
6
Caution
The equipment may be damaged if the instruction is not observed
7
Instruction for User manual
8
Do Not Reuse (Disposable medical devices)
9
Type of BF applied part
10
Manufacturer
11
Prescription Use only
Not available without a doctor’s prescription
12
Warning
13
Prohibition
Do not do anything marked with this symbol
14
Use by
It can be used up to the date stated by the manufacturer.
15
Humidity Limitations
It can be used in humidity conditions of 10 % or more and 95% or less.
16
Do not use if package is damaged
17
Temperature Limitation
18
On

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 10 / 123
4. Device description
4.1 Operation principles
This device is a patch-type electrocardiogram device that senses a signal by attaching an electrode to a
certain part of the potential difference generated when the action potentials generated when the myocardium
is active is transferred to the surface of the body.
4.1.1 What is ECG?
Like the figure 3.1, when cells or muscles move, micro-currents are generated due to micro-resistance. At
this time, each cell or muscle have action potential due to the micro-current generated by movement. The
ECG waveform is the sum of the currents that the ECG electrode detects action potential caused by
depolarization of the heart muscle and repolarization during the heartbeat. In other words, ECG is measure
of the electrical activity of the heart.
[Fig 4.1 Principle of micro-current generation]
As shown in Fig 3.2 below, the action potentials generated in the heart are gathered to view the ECG
waveform.

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 11 / 123
[Fig 4.2 ECG waveform and action potential of the heart]
4.1.2 ATP-C130 ECG measurement process
[Fig 4.3 ATP-C130 system configuration]
1) Sensor: It detects the ECG signal and receives it in a stable form, reducing the fluidity of the ECG signal
for the movement of the human body. However, offset voltage caused by electrodes occurs, and this part is
removed from 3 IN-AMP & RLD(Amplifier).
2) ESD protection circuit: It acts as a protective circuit against signals input by ESD or external static
electricity when attached to the human body.
3) IN-AMP & RLD: It detects a signal using a precision instrument amplifier for electric signals
(heart/EMG/movement) from the human body, and also uses feedback from the RLD circuit for the input of
human body’s common noise (50 Hz or 60 Hz). It plays a role in detecting a clear ECG signal by reducing
the common-mode noise. Also remove the offset voltage generated by the sensor
4) High & Low-pass filter: The role of the filter is to remove the remaining signals except the information of
the ECG signal output by the IN-AMP & RLD using a bandpass filter.
5) ECG signal amplifier: It amplifies the signal that has passed through the filter.

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 12 / 123
6)Time conversion ADC: Using the IC, It plays the role of converting analog signals into digital signals
more accurately and precisely by using internal digital filters (primary / secondary) and algorithms for
charging/discharging signals.
7) BLE IC Processor: It performs the final processing of the converted digital ECG signal using the above
Time Conversion ADC and transmits it wirelessly in BLE or stores it in the Serial NAND Flash Memory
included inside.
8) Serial NAND Flash Memory: It serves to store the digitally converted ECG signal in the internal memory.
9) BLE Wireless Communication: It plays the role of communication for wireless transmission.
10) After that, the ECG signal stored in the above process is stored in the device over 14 days, and then, the
record can be transmitted from the device to PC software installed in the PC through a dedicated cable to the
PC. The final report is documented and printed after analyzing the transmitted ECG records through
software.
4.2 Operating system diagram
No.
Item
Description
1
Power part
The part that converts the power supplied by the battery
into the proper power for the IC
2
Display part
The part that can be recognized externally according to

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 13 / 123
the result processed by the controller
3
Sensor
Part
Accelerometer
The part that senses the movement of the device
4
ECG Sensor
The part that senses electrocardiographic signal
5
Control
Part
Digital ADC
The part that converts the input signal to a digital signal
6
MCU
The part that signal processing and various control
7
Communication
Part
BLE
The part for BLE communication
8
Record part
The part that records the ECG signal
9
Antenna part
External input/output part to BLE wireless signal
10
Battery
The part that provides power to the device
11
Patch Ass’y
Patch part where ECG terminal is assembled
12
Software
F/W
S/W part that operates MCU and BLE
13
App
S/W part that processes the signal received from the
device
14
PC/SW
The part that downloads S/W stored in memory and
analyzes and reports on PC

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 14 / 123
4.3 Device and accessories
4.3.1 Device
4.3.2 Accessories
Component
Description
Picture
Dedicated Cable
After using the device (14 days),
after installing the diagnostic S/W on
the recommended PC, the device and
PC only can get the ECG record of
the device through this cable.
BLE Dongle
It is a BLE dongle to communicate
with the device from a PC that S/W
installed
USB Memory
Memory that S/W installer for
analyzing ECG records is stored.
Patch
Two extra patches are provided per
product to maintain adhesion when
the initial AT-patch is about to fall
off.

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 15 / 123
4.3.3 S/W
App (ATN-C130)
Ver 1.3.0.0 / Android 5.x or iOS
12.x or later
PC S/W (ATR-C130)
Ver 1.3.0.0 / Window10(64bit)
5. Critical Component List
Object /
part No.
Manufacturer/
trademark
Type / model
Technical data
Standard
Mark(s) of
conformity1)
PCB
ISUEXABOAR
D CO LTD,
Elim electronics
Ind.,LTD
MD1-A2-003
Flammability, V-0
IEC 60695-11-
10 UL 94
UL
ZPMV2.
E252915
FPCB
Saemon
Technology
MD1-PCB-
002
FPCB Type Antenna
CCL
SV-SS1120SFQ1
Coverlay
SV-SC015WFY1
Tape 3M 83710
-
Tested in
equipment
under
condition of
use in the ME
EQUIPMENT
Case
SON
TECHNOLOG
Y., LTD
MD1-A1-001
MD1-A2-001
MD1-A2-002
LG Chems
SC1004A
Flammability, HB
IEC 60695-11-
10 UL94
UL
QMFZ2.
E67171
Patch
HANSUNG
COLOR CO.,
LTD
MD1-A1-004
Medical Grade PU
Tape
95 x 52.6 x 0.04mm
Medical Grade
Silicone Tape
72 x 36.4 x 0.25 mm
Gel Type Medical
Hydrogel CRRA240
Φ12 x 0.5mm
-
Test in
equipment
under
condition of
use in the ME
EQUIPMENT
Battery
RENATA
CR2032
3.0V_235mAH
3.2mm H x 20mm D
IEC 60086-4
DK-74955-
UL
Panasonic
CR2032
3.0V_235mAH
3.2mm H x 20mm D
IEC 60086-4
UL1642

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.10.30
Revision No.
0
Page 16 / 123
Object /
part No.
Manufacturer/
trademark
Type / model
Technical data
Standard
Mark(s) of
conformity1)
Supplementary information:
1) Indicates a mark which assures the agreed level of surveillance. See Licenses and
Certificates of Conformity for verification.
6. Patient Contact Part, Sterilization and Reusable
Part name
Patient Contact
Part
Patient Contact
Duration
Sterilization
Reusable
PU Tape
Skin
24h < D ≤30 days
X
Single-use
Hydrogel
Skin
24h < D ≤30 days
X
Single-use
Silicone Tape
Skin
24h < D ≤30 days
X
Single-use
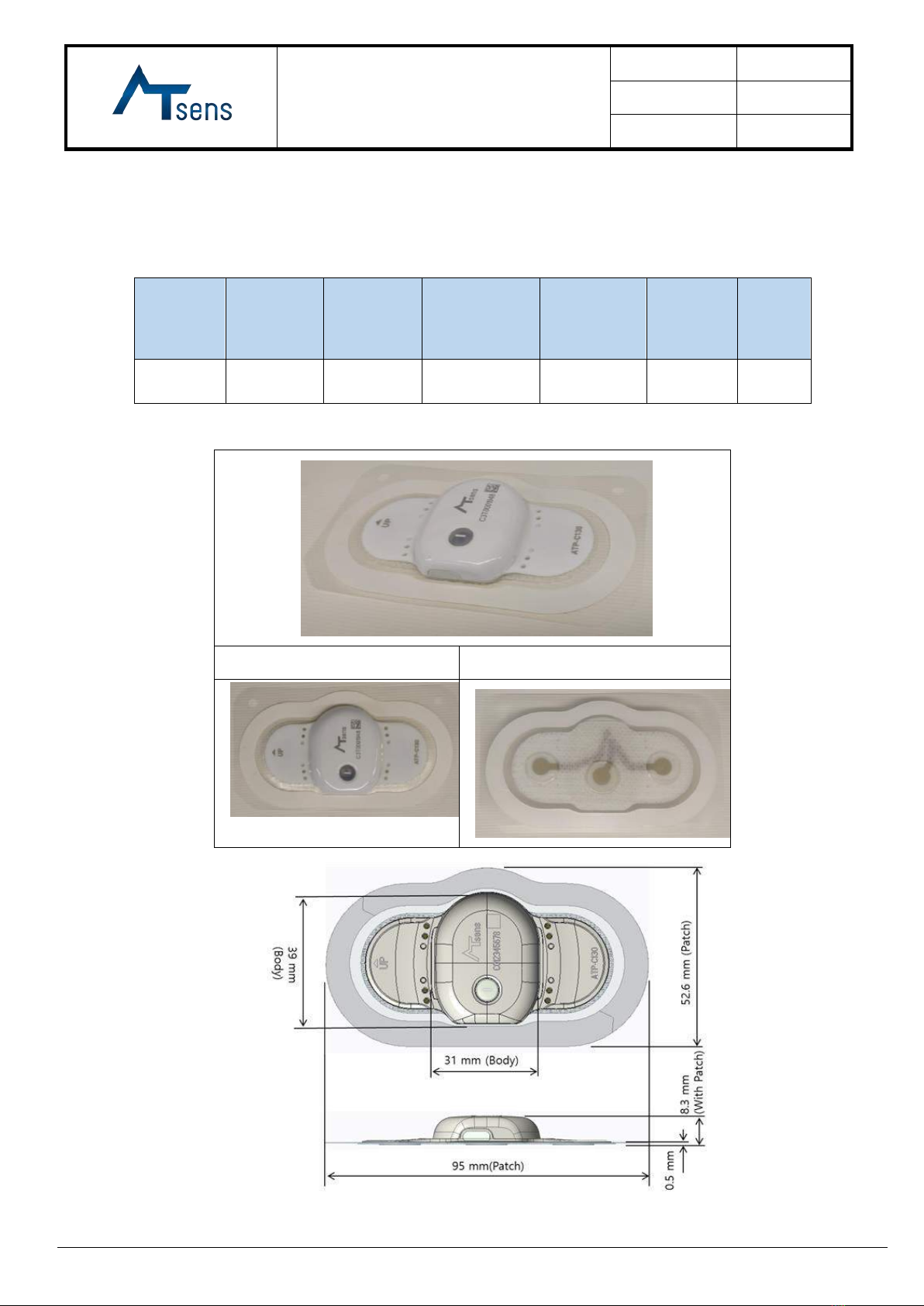
The entire bottom part makes contact with the patient’s skin.
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.08.30
Revision No.
0
QF-001
ATsens Co., Ltd.
17/123
7. Photograph and/or Drawing of the Device
7.1 External Shape
1) Device (Model: ATP-C130)
Body
width(W)
Body
length(L1)
Patch
length(L2)
Patch
Width(W2)
Patch
thickness(T)
Total
height(H)
Weight
39 mm
31 mm
95 mm
52.6 mm
0.5 mm
8.3 mm
12.6(g)
Front View
Rear View
2) Dedicated cable length: 1 (m)
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.08.30
Revision No.
0
QF-001
ATsens Co., Ltd.
18/123
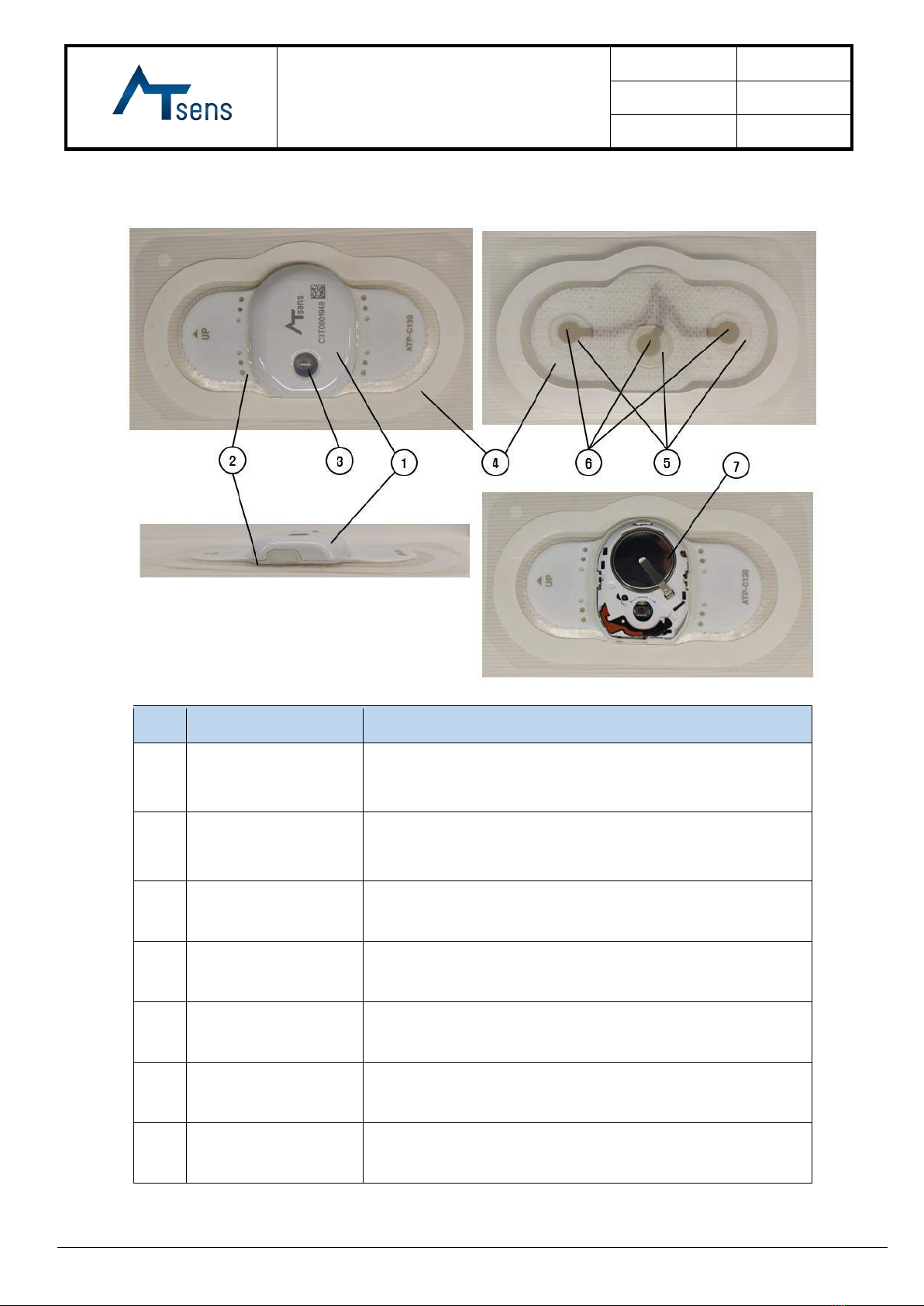
7.2 Interface Description
No.
Name
Description
①
Case Top
The power button is assembled, and the company logo and S/N
of the device are printed on the surface.
②
Case BTM
It is made of PC and TPU double injection material and has an
electrocardiogram electrode.
③
Power Button / LED
The power button turns on the power and you can check the
power status through the LED.
④
Patch Ass'y
It uses medical grade tape and adheres to the skin surface.
⑤
Hydrogel
It is located between the ECG electrode and the skin, and it has
a function that allows constant measurement of ECG signals.
⑥
Electrodes
ECG electrode
⑦
Battery
Coin Battery/CR2032
User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.08.30
Revision No.
0
QF-001
ATsens Co., Ltd.
19/123
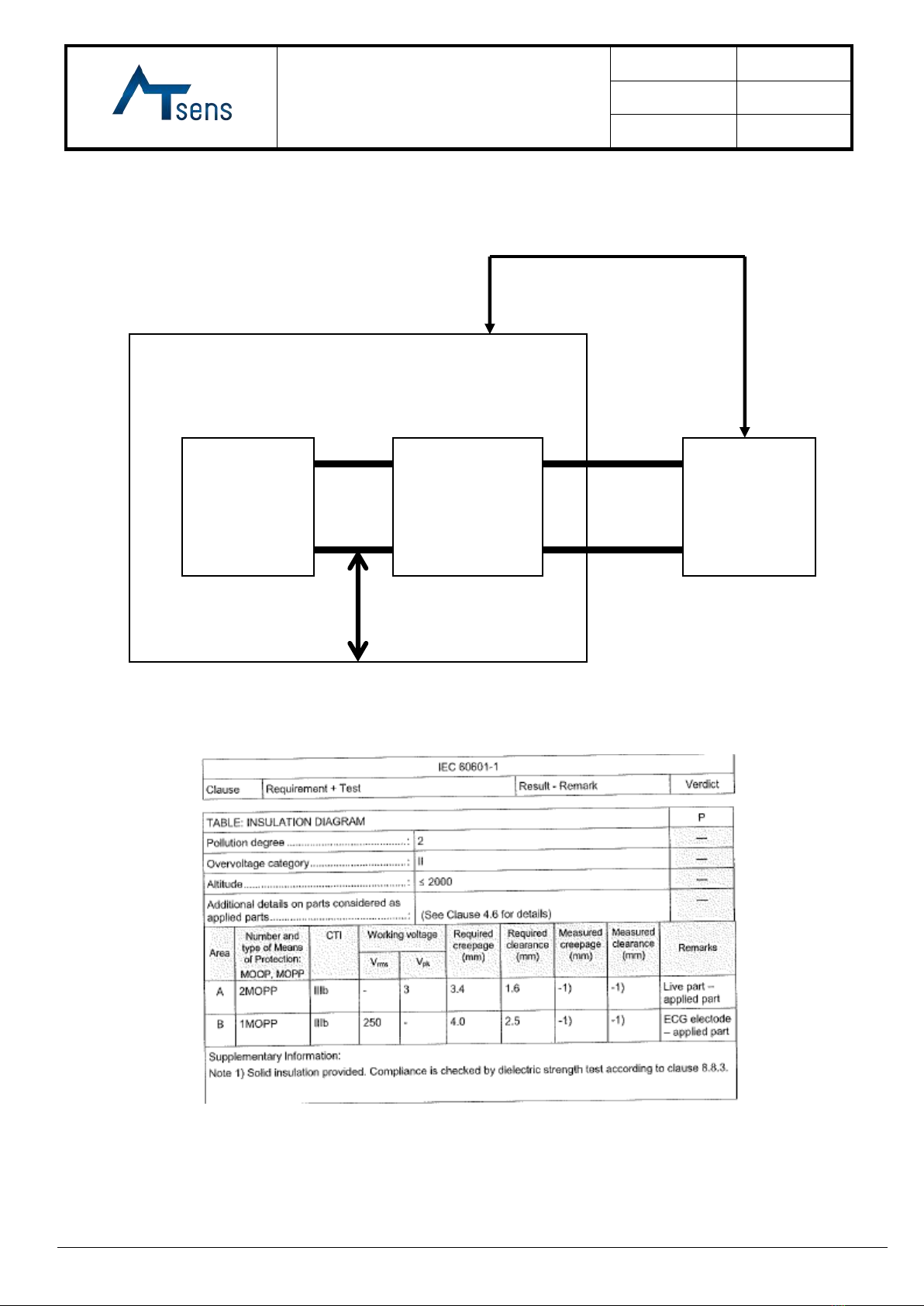
8. Insulation
Insulation is designed according to IEC 60601-1:
Main Control
Unit
CR2032
Primary
Lithium
Battery
Electrode
Secondary Circuits
Plastic Enclosure
A
B
Entire enclosure of device is regarded as BF type applied part

User Manual
Document No.
UM-C-002-EN
Date of Revision
2020.08.30
Revision No.
0
QF-001
ATsens Co., Ltd.
20/123
9. How to use (Device,App & PC S/W)
9.1App (ATN-C130) Icon Screen and Initial Launch Screen
Figure 9.1 AT-note App Icon Figure 9.2 Intro Screen 1 Figure 9.3 Intro Screen 2 Figure 9.4 Intro Screen 3
Table 9.1.
No
Item
Description
1
Run App (ATN-C130) Icon
Click the icon to run the App (ATN-C130)
2
Intro Screen 1
When running App (ATN-C130), the relevant intro screen is
displayed for about 2 seconds and then the screen is switched
to the next screen.
3
Intro Screen 2
After the intro screen is displayed for about 1 second, the
screen changes to the next screen.
4
Intro Screen 3
Intro Screen
5
Contents of the Device
Connection Guide
Contents of the guide for device connection
6
Screen for Device
Connection navigation
button
Screen call button for device connection
Table of contents
Other ATsens Medical Equipment manuals