3
Contents
Important Information/Reminders ................................................................................................ 1
Introduction .................................................................................................................................. 4
Definitions..................................................................................................................................... 4
Compliance ................................................................................................................................... 4
Intended Use................................................................................................................................. 4
Contraindications .......................................................................................................................... 5
Parts and Accessories .................................................................................................................... 5
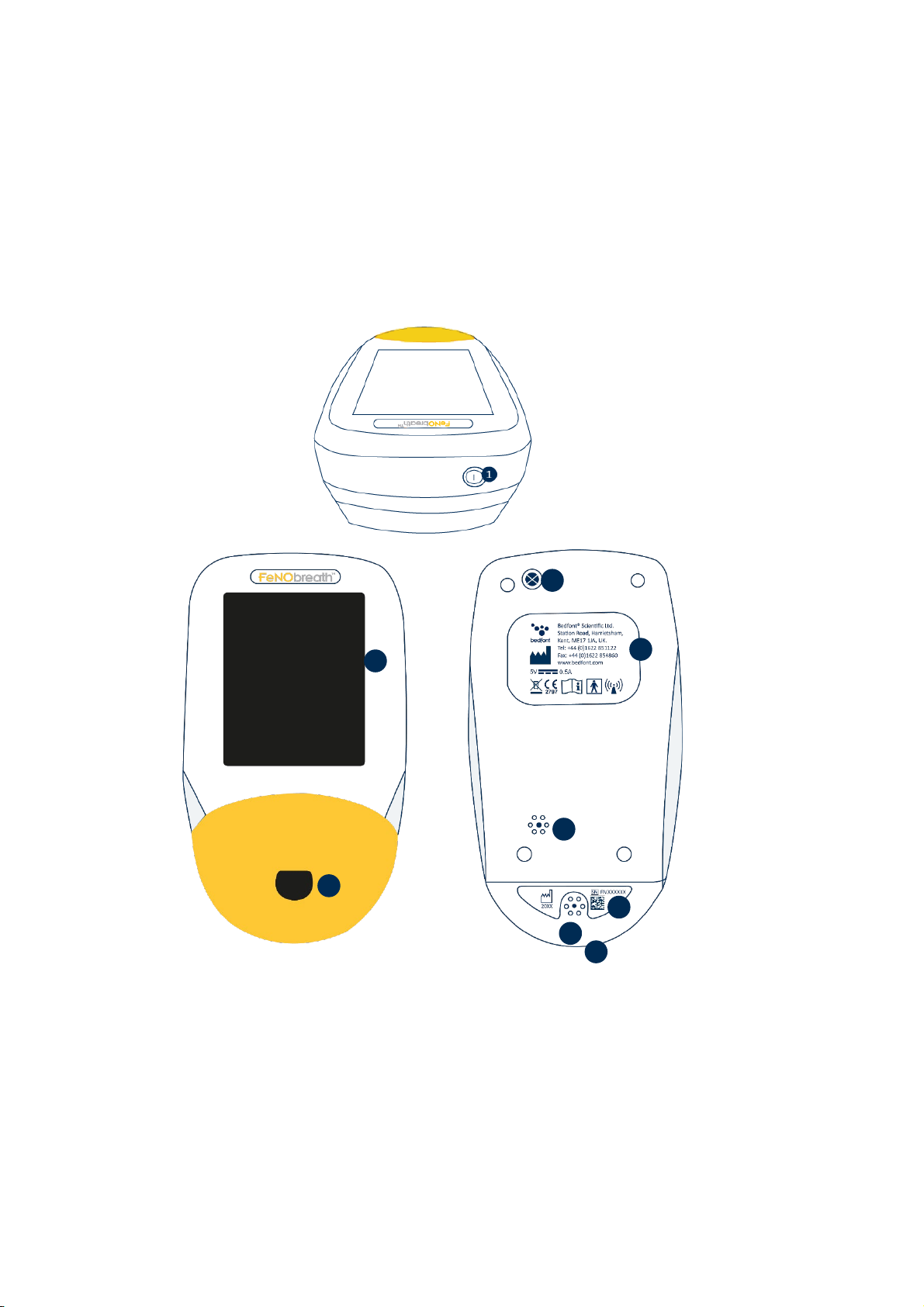
Instrument Layout ......................................................................................................................... 6
Installation and Set-up................................................................................................................... 7
User Interface................................................................................................................................ 9
Demo mode................................................................................................................................. 10
Performing a Breath Test............................................................................................................. 13
Patient profiles ............................................................................................................................ 15
Maintenance ............................................................................................................................... 19
Settings ....................................................................................................................................... 21
Data Reset................................................................................................................................... 30
Quality Check and Calibration...................................................................................................... 32
Calibration using a CaliBag®......................................................................................................... 41
Cybersecurity .............................................................................................................................. 45
Technical Specification ................................................................................................................ 46
Buttons Explained........................................................................................................................ 47
Troubleshooting .......................................................................................................................... 48
Glossary of Symbols and Safety Information ................................................................................ 53
Wireless ...................................................................................................................................... 55
Emissions..................................................................................................................................... 57
Immunity..................................................................................................................................... 57
Summary of the clinical data........................................................................................................ 59
Analytical Performance Data ....................................................................................................... 61
Interpretation of FeNO Values ..................................................................................................... 62
Warranty ..................................................................................................................................... 62
Returns........................................................................................................................................ 63