Bemer BEMER-SET PRO User manual
–
www.bemergroup.com
-
:: User Manual
3 Years Warranty
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 11960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 1 04.11.2020 08:47:2304.11.2020 08:47:23

:
.
Austrasse 15 //9495 Triesen
Liechtenstein
Tel. +423 399 39 99
info@bemergroup.com
..
Congratulations on the purchase of your new BEMER set. These high quality products are manufactured by us with the greatest
of care and meet our high quality standards, as well as the European and international production standards of medical products.
Not only is this device of the highest quality, but it is also user friendly, with optimal functionality determined by our satisfied
customers over the past 10 years.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 21960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 2 04.11.2020 08:47:2304.11.2020 08:47:23

Contents
Scope of Delivery 5
Foreword 6
Safety Instructions 7
Intended Use 9
Contraindications 9
Precautions 12
User Information 14
Basic Plan 16
General Information 19
Examples of Application 20
Connections – B.BOX 22
Start-up Procedure – B.BOX 23
Connection – Applicator Module 23
Display – B.BOX Professional 24
Applications – B.BOX Professional 26
Default Settings – using the B.BOX Professional 28
Updates 31
B.SCAN 32
Wall Mount Assembly 33
Rechargeable battery – B.BOX 34
Cleaning 36
Maintenance 37
Proper Disposal 37
Troubleshooting 38
Technical data 40
Manufacturer‘s Declaration of Conformity 44
Warranty 50
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 31960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 3 04.11.2020 08:47:2304.11.2020 08:47:23

1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 41960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 4 04.11.2020 08:47:2304.11.2020 08:47:23

. .
B.GRIP (holder module for B.SPOT)
[Art. No.
431000
B.SPOT (application module
for local treatment)
[Art. No.
431101
B.PAD (flexible application module
for local treatment)
[Art. No.
430301
B.SCAN (scanning module for
functional checks)
[Art. No.
450100
Wall mount
[Art. No.
450200]
Vehicle connection
cable
[Art. No.
440300]
Attachment belt
(B.GRIP)
[Art. No.
450700]
B.BOX Professional
(control device with multitouch display)
Scope of Delivery – Pro-Set* [Art. No. 410201]
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 51960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 5 04.11.2020 08:47:2604.11.2020 08:47:26

6
Symbols found in the operating instructions
Warning/Caution – failure to observe
can result in possible injuries or property
damage.
Proper disposal
Tip
Manufacturer
CE conformity marking according to
Medical Devices Directive 93/42/EWG
Temperature limit
Air pressure limit
Relative humidity limit
Foreword
This instruction manual belongs to this device. It contains important informa-
tion for usage and handling. Read these instructions completely. Non-obser-
vance of these instructions can lead to injury of the user or damage to the unit.
Device symbols
CE conformity marking according to
Medical Devices Directive 93/42/EWG
Proper disposal
Manufacturer
Please refer to the user manual
Protection class
Device is of protection class II
Application part type
Application part is type BF
Serial number
Refer to instruction manual/ booklet
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 61960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 6 04.11.2020 08:47:2604.11.2020 08:47:26

7
Safety instructions
Device symbols
CE conformity marking according to
Medical Devices Directive 93/42/EWG
Proper disposal
Manufacturer
Please refer to the user manual
Protection class
Device is of protection class II
Application part type
Application part is type BF
Serial number
Refer to instruction manual/ booklet
•
Consult with your doctor before using BEMER devices, if you are in the care
of a doctor.
•
Before you connect the BEMER device to the power supply, please make sure
that the power supply is identical with that of the B.BOX power pack supply.
•
Power cable and power plug must be in their original, undamaged condition.
Immediately replace any damaged parts!
•
Make sure to unplug the power plug when cleaning!
•
Do not misuse BEMER devices! The devices may not be used for any purposes
other than those mentioned here.
BEMER products are manufactured according to the latest tech-
nology and are completely safe to operate. Nevertheless, hazards
could be caused by this device. This is particularly the case when
insufficiently trained personnel are operating the device, or should
the device be inappropriately used and not according to its indica-
tions for use. Make sure that you have completely read and under-
stood the instruction manual. If you have any questions, do not
hesitate to contact us.
Direct contact between the device and its application modules
is only permissible on undamaged skin.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 71960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 7 04.11.2020 08:47:2604.11.2020 08:47:26

8
•
Always keep BEMER devices in proper working order.
•
Only use approved BEMER accessories.
•
Please check BEMER devices on a regular basis for damage.
Immediately replace any damaged parts.
•
Please read the user manual before use.
•
BEMER devices may only be used on a stable, non yielding
surface (e. g. floor, sofa, bed, table).
•
BEMER devices are not toys. Keep pets away from devices.
•
The BEMER device is not intended to be used for use by
people under 18 years of age.
•
When using please make sure that the applicator connection
cables are arranged in such a way that there can be no strangu-
lation or restriction of breathing.
•
Before somebody else uses the device, the unit and its
components must be cleaned.
•
Caution when expanding or collapsing the top part of the
instrument (see chapter Connections – B.BOX).
•
Do not modify this equipment without authorization from
the manufacturer.
•
Local laws and regulations must be observed by professional users.
•
If you experience adverse reactions, stop using BEMER devices
and consult your medical doctor.
•
Stimulation should not be applied over the carotid sinus nerves,
particularly in patients with a known sensitivity to the carotid
sinus reflex.
•
Stimulation should not be applied over the neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may
occur and the contractions may be strong enough to close
the airway or cause difficulty in breathing.
•
Stimulation should not be applied transthoracically (through
chest) in that the introduction of electro-magnetic stimula-
tion into the heart may cause cardiac arrhythmias.
•
Stimulation should not be applied transcerebrally (through
the head).
•
Stimulation should not be applied over infected, or inflamed
areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose
veins, etc.
•
The devices are not intended to be used during sleep.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 81960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 8 04.11.2020 08:47:2604.11.2020 08:47:26

9
Intended Use Contraindications
•
To temporarily increase local blood circulation in healthy
leg muscles
•
To stimulate healthy muscles in order to improve and facilitate
muscle performance.
The following absolute contraindications must be observed:
•
Immunosuppressive therapy in consequence of transplantation
•
Immunosuppressive therapy in consequence of allogenic cellular
transplantations or bone marrow stem cell transplantation
•
Other conditions often requiring immunosuppressive therapy,
e.g. autoimmune diseases or dermatological diseases are not
contraindications to the use of BEMER therapy. BEMER therapy
application has to be cleared by physician in charge.
•
Do not use the device if you have a diagnosed
Deep Vein Thrombosis (DVT)
•
Active medical implants that lead to stimulation (e.g. pacemakers,
defibrillators, brain stimulators, muscle stimulators) represent
a relative contraindication. The adjuvant application of the
BEMER therapy must be discussed with the patient's attending
physician.
•
All active medical implants that are intended to administer
medication (medication pumps) are an absolute contraindication
and prohibit the use of BEMER therapy.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 91960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 9 04.11.2020 08:47:2604.11.2020 08:47:26

10
BEMER products may not be used for any pur-
pose other than that described in this chapter.
Use of the device beyond that which has been
specified is considered contrary to the intended
purpose. The manufacturer is not responsible
for any damages resulting from improper use.
The user is solely responsible for any risks.
The BEMER may be used by adults (over 18
years old).
This system is not intended to be used by users
with restricted physical, sensory or mental
capabilities or lack of experience and/or lack
of knowledge, unless they are supervised by
a person responsible for their safety, or receive
instructions from someone on how to use the
device.
Warnings
With the following conditions, it is strongly recommended
that you consult a medical doctor prior to the use of BEMER
therapy:
•
Do not use on head and face
•
Fever of unknown origin
•
Infectious diseases
•
Caution should be used for patients with severe cardiac rhythm
disorders and active implants such as cardiac pacemakers and
insulin pumps.
•
Caution should be used for patients with cardiac pacemaker,
implanted defibrilator, or other implanted metallic or electronic
devices.
•
Severe psychoses
•
Non-controlled seizure disorders (e.g. epilepsy)
•
Long-term use of β-recepter antagonists (Beta-Blockers)
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 101960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 10 04.11.2020 08:47:2604.11.2020 08:47:26

11
•
Long-term use of corticoid agents (corticosteroids)
•
Long-term use of cumarin derivates (Warfarin / Coumadin)
Other anticoagulant agents such as non-steroidal drugs like
diclofenac or other anti-hypertonic drugs such as calcium
antagonists and angiotensin-receptor-antagonists are not
known to interact with the BEMER therapy.
•
For users suffering from tumor diseases or other serious diseas-
es that require ongoing medical treatment and/or medication,
the complementary application of BEMER therapy has to be
discussed with the treating medical doctor.
•
If you feel any discomfort while using BEMER immediatley stop
all applications and consult your physician in charge. Further
applications of BEMER have to be cleared by him or her.
Warning
Portable RF communications equipment (including periph-
erals such as antenna cables and external antennas) should
be used no closer than 30 cm (12 inches) to any part of the
BEMER Pro Set or BEMER Classic Set, including cables
specified by the manufacturer. Otherwise, degradation of
the performance of this equipment could result.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 111960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 11 04.11.2020 08:47:2604.11.2020 08:47:26

12
•
Complaints, symptoms or diseases must be clarified by a medical
doctor prior to the application of BEMER therapy
•
Prescribed medications may only be altered after consulting
the treating physician
•
In users who regularly take blood thinning or clotting inhibitor
medications, close monitoring of the clotting factors by the
treating medical doctor are strongly recommended prior to
starting BEMER therapy. BEMER therapy may intensify or weak-
en the effect of such drugs.
•
In case of questions regarding BEMER therapy, the treating
medical doctor is advised to contact BEMER medical support
•
In general, application of BEMER therapy in users with active
electronic implants and devices (such as medications pumps,
neurostimulators etc) has to be discussed and cleared by the
treating medical doctor.
Precautions
•
BEMER therapy is an important complementary therapy option
and is not intended to replace other treatments
•
Some patients may experience skin irritation or hypersensitivity
due to the electrical stimulation.
•
Powered muscle stimulators should be kept out of the reach
of children.
•
Caution should be used in the presence of the following:
a. Over the menstruating or pregnant uterus; and
b. Over areas of the skin which lack normal sensation.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 121960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 12 04.11.2020 08:47:2704.11.2020 08:47:27

13
Warning
Use of this equipment adjacent to or stacked
with other equipment should be avoided be-
cause it could result in improper operation. If
such use is necessary, this equipment and the
other equipment should be observed to veri-
fy that they are operating normally.
Warning
Use of accessories, transducers and cables
other than those specified or provided by
the manufacturer of this equipment could
result in increased electromagnetic emissions
or decreased electromagnetic immunity of
this equipment and result in improper op-
eration.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 131960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 13 04.11.2020 08:47:2704.11.2020 08:47:27

14
These operating instructions contain important
information. Keep them close to your device in
order to inform yourself about safety instructions
as well as proper handling.
Please read the operating instructions very care-
fully before using the device. In this way you can
be assured that you benefit from all the advantages
that this system has to offer and protect yourself
and others from harm.
BEMER devices are designated only for the described,
intended use according to the operating instructions.
The BEMER temporarily increases local blood
circulation in healthy leg muscles and stimulates
healthy muscles in order to improve and facilitate
muscle performance.
The BEMER devices may be used for the following:
• To reduce stress
• To support sleep management
Treatment principles
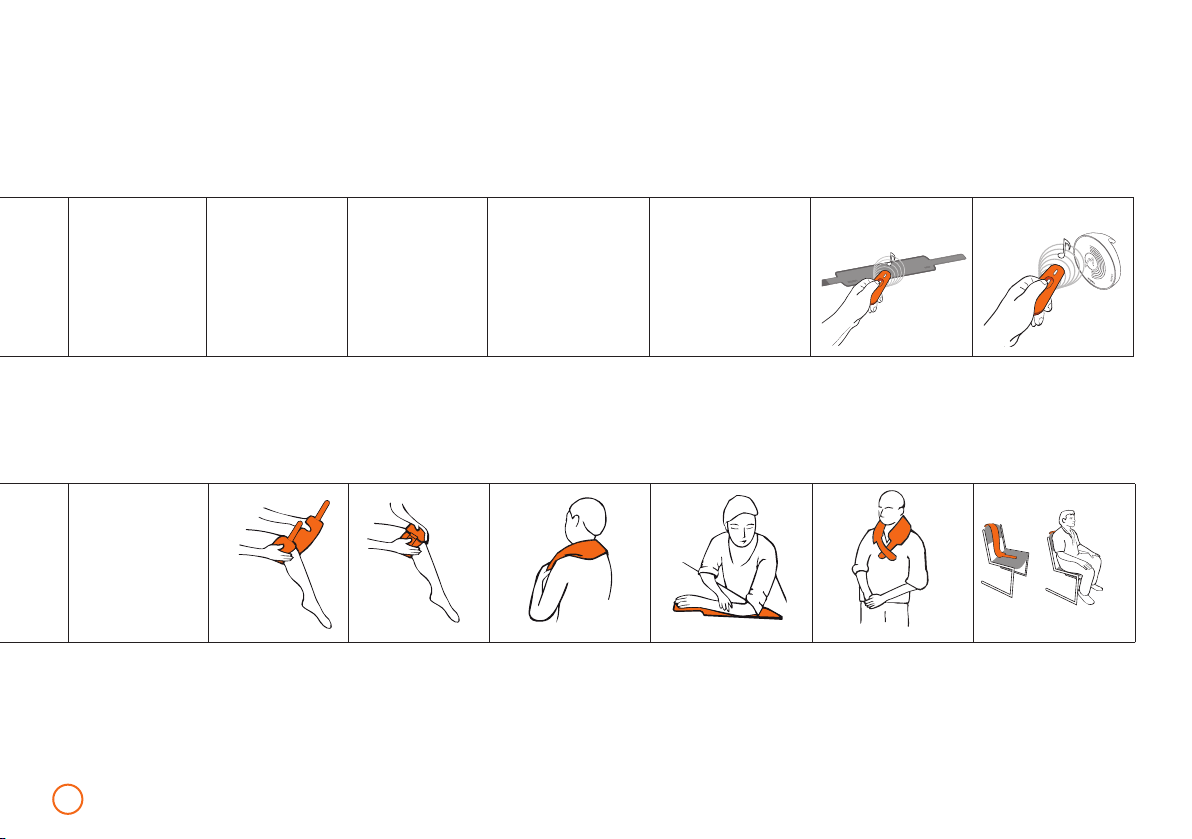
1. Local treatment
The application modules B.SPOT and B.PAD are intended
for the local treatment of individual body parts, working with
a maximum flux density of 150 T. The targeted application
should follow the application of the basic plan and should be
applied to the shoulders, waist, back and lower extremities.
B.SPOT is an intensively active application module for use in
local treatment. B.SPOT is attached to the universal B.GRIP hold-
er and held on the body area that is to be treated. Alternatively,
the holder and application module can also be attached to the
part of the body that is in need of treatment with the provided
attachment strap.
B.PAD is an intensively active application module for local treat-
ment on individual body parts. The B.PAD is especially attractive
because of its flexibility and can be attached with the help of a
Velcro fastener strap to nearly every conceivable part of the body.
When using on disabled or mobility impaired people, the B.PAD
can provide a particularly valuable service.
User Information
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 141960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 14 04.11.2020 08:47:2704.11.2020 08:47:27

15
2. Programs and intensity levels
The control devices B.BOX Professional offer 10 different inten-
sity levels and three pre-defined programs. The intensity levels
are applied during the generalized local treatment according to
the basic plan, while the programs P1 – P3 are carried out in a
step-by-step manner within the scope of the local treatment.
The existing, predefined programs cover the wide range of
application requirements needed for daily use.
Frequency of application
Whenever possible, BEMER therapy should be carried out on
a daily basis. Intermittent applications mean a lessened effect,
resulting in longer application durations.
There is no hard and fast rule for the distribution of applications
throughout the day. It depends on the therapy goal and the
situation of the individual user.
•
For prevention, maintaining overall health, and supporting the
body’s performance, we recommend to use two applications
a day following the Basic Plan on page 17.
•
To treat acute stimulation targeted with the application
module, we recommend the additional use of 2-3 local appli-
cations with the programs P1-P3 additionaly to the basic
Plan. Overdosing or habituation effects from using the BEMER
system are not known.
•
At home, applications can be customized according to the indi-
vidual’s situation and needs. If possible, a treatment should be
performed both in the morning and in the evening.
•
At the doctor’s office or therapeutic practices, it is usually possi-
ble to have only one application per day. Usually this consists of
a local treatment with the B.PAD and, when necessary, this is
immediately followed by another treatment with an applica-
tion module for localized treatment (double treatment).
•
Do not use two different local treatments at the same time,
on the same user.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 151960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 15 04.11.2020 08:47:2704.11.2020 08:47:27

16
Practical tips for application
The effects of the specific signal configuration are
scientifically proven and essentially independent
of external influences. Nevertheless, the effects of
the application can be optimized by taking into
account these few tips:
•
Make sure you find a comfortable position.
•
Avoid wearing tight or constricting clothing.
•
Choose a room with a comfortable
temperature for your therapy.
•
Avoid stress or distraction.
•
Avoid drinking coffee or tea for at least one hour
before and after the treatment.
•
Avoid consuming tobacco products for at least
one hour before and after the treatment.
•
Drink a glass of still water before and
after application.
Basic Plan
The Basic Plan is a local therapy that stimulates healthy muscles
in order to improve and facilitate muscle performance. This
is the basis of treatment with BEMER therapy and defines the
standard of treatment for conditions of reduced well-being.
The Basic Plan treatment (BP treatment) with the B.PAD should
generally be carried out 2x daily, each session has a duration of
8 minutes (see “Short treatments” for B.BOX Pro described on
page 26).
The first cycle lasts 6 weeks, the following ones only 4 weeks.
Begin with level 1 and increase a level each week up to level 6.
After the 7th week, begin the new cycle starting with level 3.
For ongoing care to support the musculature, the “LOW” setting
is recommended for usage with the “Basic Plan” (see “Default
settings” for B.BOX Pro described on page 28).
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 161960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 16 04.11.2020 08:47:2704.11.2020 08:47:27

17
Level 1 plus Level 2 plus Level 3 plus Level 4 plus Level 5 plus Level 6 plus
Level 1 Level 2 Level 3 Level 4 Level 5 Level 6
Level 3 plus Level 4 plus Level 5 plus Level 6 plus
Level 3 Level 4 Level 5 Level 6
Basic Plan (treatment plan for local treatment)
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 171960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 17 04.11.2020 08:47:2704.11.2020 08:47:27

Programs on the Control Unit
The predefined program settings were specially developed for
the use of local applicators (B.SPOT, B.PAD) so that an addi-
tional therapy impulse can be given locally.
Local application is not a sufficient replacement for the
Basic Plan.
Use the local application two or three times per day, beginning
with P1 and change after two or three days to P2. The aim is not
to get to P2, if you feel uncomfortable with P2, return to P1.
P1
recommended
for home use 8 min.
Low intensity for
superficial /
minor stimulation
P2
recommended for
health care facilities 16 min.
Middle intensity for
somewhat deeper /
moderate stimulation
P1-P2 for daily users
P3 20 min.
Strong intensity for
muscle stimulation
and training
P3 for active athletes
For local application using the pre-defined programs, the
“HIGH” setting is recommended for use (see “Default settings”
for B.BOX Pro described on page 28).
BEMER Usage Support
If you still have questions or are uncertain, please
contact your treating medical doctor or contact
BEMER medical support:
Email: usage-support@bemer.services
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 181960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 18 04.11.2020 08:47:2704.11.2020 08:47:27

19
Both the Basic Plan and all other application sug-
gestions are based on many years of experience.
This experience has also proven that regular ap-
plications are more important than the optimal
choice of intensity. Successful therapy results are
achieved most quickly through regular applica-
tion. However the duration of the applications
depends on how difficult the problem and for
how long the body’s own regulatory systems
have been impaired.
General Information
The Basic Plan treatment is carried out using the B.PAD ac-
cording to the basic plan using intensities 1-6 (short applica-
tion). Local treatments are carried out using the application
modules B.SPOT and B.PAD and are used depending on the
desired depth of penetration with the use of the predefined
programs (P1-P3).
General tips
•
Please note that several application modules can be
connected to the B.BOX.
•
Basic plan treatments always last 8 minutes,
regardless of the chosen intensity.
•
Programs have different durations and
use varying intensities (see page 18).
•
Ongoing treatments can be canceled at any time
by pressing the power button [START/ STOP].
•
Canceled treatments can not be continued;
they must be restarted.
•
The plus Signal is switched on by default
and can be switched off if needed.
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 191960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 19 04.11.2020 08:47:2704.11.2020 08:47:27
20
B.SCAN
The B.BOX has an integrated test device for checking
the performance of your application module.
B.PAD
Handy, flexible and universally mobile.
This application module is made for local treatment.
Examples of Application
1960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 201960-2002-BEDIENANL-B.BOX-USA-FDA-2020-US-en-v13-Akku Rev20_4.indd 20 04.11.2020 08:47:2704.11.2020 08:47:27
This manual suits for next models
1
Table of contents
Other Bemer Personal Care Product manuals
Popular Personal Care Product manuals by other brands
Breathings
Breathings Bulo instruction manual

Braun
Braun Oral-B AdvancePower WaterJet MD 31 user guide

Semilac
Semilac PRO WHITE instruction manual

American Standard
American Standard Elongated Toilet Seat 5284.016 Specification sheet

Bioventus
Bioventus EXOGEN Quick instruction guide

Somnetics
Somnetics Transcend 3 miniCPAP user manual