Bemer Therapy System Evo User manual

BEMER Therapy System Evo
System for targeted and full-body therapy
by means of magnetic fields and light
Instructions for use
More Health. More Life. 23.1
bemergroup.com
EN | HU | CZ | SK | PL | SR | RO

Translation of the original instructions for use
BEMER Int. AG
Austrasse 15
LI-9495 Triesen
www.bemergroup.com
Tel.:+423 399 39 99
Fax:+423 399 39 98
Collection ID 3448, Version 1
Date: 11/2022
Table of Contents
1. General information 6
1.1 Instructions for use 7
1.2 Symbols 8
1.3 Liability 9
1.4 Copyright notice 9
1.5 Warranty 10
1.6 Notification of incidents 10
2. Safety 11
2.1 Intended use 11
2.1.1 Intended purpose 11
2.1.2 Medical applications 11
2.1.3 Indications for use 11
2.1.4 Contraindications 12
2.1.5 Potential side effects 12
2.1.6 Intended use 13
2.1.7 Intended users 13
2.1.8 Intended patient group 14
2.1.9 Body regions 14
2.1.10 Intended environment and field of application 14
2.2 Clinical benefits 14
2.3 General safety instructions 15
2.4 Medical notes 20
2.4.1 Medical notes on PEMF 20
2.4.2 Medical notes on LLLT 21
2.5 Notes for professional users of medical products 22
2.6 Safety labels 22
2.7 Protective equipment 23
2.8 Environmental protection 23
3. Technical data 24
3.1 Storage, transport, and operating conditions 24
3.2 Product-specific technical data 25
3.3 EMC conformity 32
Instruction for use
RO SR PL SK CZ HU EN
32

4. BEMER Therapy System Evo 37
4.1 System description 37
4.2 System overview 38
4.3 Scope of delivery for sets, packages, and individual products 40
4.4 Main components of the BEMER Therapy System Evo 44
4.5 Applicators/application modules and their use 46
4.5.1 B.Body Evo (PEMF) – full-body applicator for universal use 46
4.5.2 B.Bed Evo (PEMF) – full-body applicator for use in bed 46
4.5.3 B.Pad Evo (PEMF) – universal applicator for targeted treatment 47
4.5.4 B.Spot Evo (PEMF) – applicator module for targeted treatment 48
4.5.5 B.Sit Evo (PEMF) – applicator for targeted treatment while sitting 48
4.5.6 B.Light Clear Evo/B.Light Restore Evo (LLLT) – for targeted light therapy 48
4.5.7 B.Grip Evo interchangeable adapter 49
4.6 Accessories 50
4.6.1 B.Box Evo Battery Stand 50
4.6.2 B.GripEvo Attachment Strap 50
4.6.3 B.Box EvoPower Supply 51
4.6.4 B.BoxEvo Car Adapter 51
4.6.5 B.Light Evo Safety Glasses 52
4.6.6 B.Box Evo Wall Mount 52
5. Transport and storage 53
5.1 Safety 53
5.2 Symbols on the packaging 53
5.3 Storage of the packaging 53
6. Recommended use 54
6.1 Recommendations for application of magnetic field therapy (PEMF) 54
6.1.1 The plus Signal 54
6.1.2 Full-body therapy 54
6.1.2.1 Intensity modes and sleep program (basic plan) 54
6.1.2.2 Sleep program 56
6.1.3 Targeted treatment 56
6.2 Recommended use of light therapy (LLLT) 57
6.2.1 B.Light Clear Evo 58
6.2.2 B.Light Restore Evo 58
7. Commissioning 59
7.1 Connecting the B.Box Evo Stand, Wall Mount, and Battery Stand 59
7.2 Connecting the power supply 60
7.3 Connecting the applicators and application modules 60
7.4 Switching on the B.Box Evo 60
8. Using the BEMER Therapy System Evo 62
8.1 Switch the unit on (start screen) 62
8.2 Main menu 63
8.2.1 Status bar 63
8.2.2 Settings (start screen) 64
8.2.3 Adjust the signal volume 64
8.2.4 Adjust the display brightness 65
8.2.5 Set the current time 66
8.2.6 Expert mode editor 66
8.2.7 System 67
8.2.7.1 Switch the ambient light on/off 68
8.2.7.2 Enable / disable energy saving mode 68
8.2.7.3 Select system language 68
8.2.7.4 Set the time format 68
8.2.7.5 System information 69
8.3 Main menu 69
8.3.1 Intensity mode 70
8.3.2 Program mode 71
8.3.3 Sleep program 72
8.3.4 Light therapy 74
9. Cleaning and care 75
9.1 Cleaning 75
9.2 Disinfection 76
10. Disposal 77
11. Error messages and remedies 78
12. Meaning of the symbols on the labels (devices and packaging) 80
RO SR PL SK CZ HU EN
54
1. General information
Thank you for purchasing our BEMER Therapy System Evo and for the confidence you have placed
in us. The BEMER Therapy System Evo is a versatile and flexible product thanks to the various
applicators. The BEMER Therapy System Evo is your daily companion, regardless of whether your
goal is to prevent illness and maintain an active lifestyle or to support a prescribed course of
treatment. (Please also refer to the information in chapter 2.)
Please read these instructions for use carefully before using the system for the first time. By
doing so, you will avoid damage to the device and ensure that the warranty
is not invalidated.
When purchasing the BEMER Therapy System Evo, please ensure that you are given an introduction
to the system by an official and certified BEMER partner.
If you have further questions or require training, our customer service team will be happy to assist you.
The original language of these instructions for use is German.
1.1 Instructions for use
These instructions for use are an integral part of the BEMER Therapy System Evo. They enable
the user to use the BEMER Therapy System Evo safely and efficiently.
The user must have carefully read and understood these instructions for use before com-
missioning the system. The basic prerequisite for safe use is compliance with all specified
safety instructions.
In addition to the information contained in these instructions for use, the local accident prevention
regulations and occupational safety regulations apply.
These instructions for use must be kept in the immediate vicinity of the BEMER Therapy System
Evo and within reach of the operator at all times.
Product images
Your BEMER Therapy System Evo may differ from the illustrations in this document. However, all
descriptions are designed to apply analogously. If components are described that are not included
in the scope of delivery, they are marked as optional.
Brand names and trademarks
Product and / or company names mentioned in these instructions for use may be registered
trademarks of the respective companies.
Gender-specific wording
For easier readability, masculine nouns/pronouns are sometimes used in this document. However,
its content applies equally to all readers regardless of their sex or gender.
RO SR PL SK CZ HU EN
76

1.2 Symbols
NOTE indicates a potential hazard which,
unless avoided, could result in damage to the product and / or data loss.
NOTE
CAUTION indicates a potential hazard which,
unless avoided, could result in minor injuries.
CAUTION
WARNING indicates a potential hazard which,
unless avoided, could result in severe injuries or even death.
WARNING
DANGER indicates an imminent hazard which,
unless avoided, could result in severe injuries or even death.
DANGER
Warning sign
Safety symbol that indicates a risk or hazard.
Command sign
Safety symbol that prescribes a specific action.
Prohibition sign
Safety symbol that indicates a prohibited action.
Information
Indicates user tips and generally useful information for
optimal use of the product.
Use of symbols Description Example
zA bullet point describes an
action (activity)
zSwitch on the device.
The result of an action
(activity)
e.g., a new operating
window opens
—List – in no
particular order
―B.Box Evo
―B.Box Evo
―...
(Cross reference) Reference to a chapter or page (chapter 5.1)
<BUTTON> Refers to the actuation of
an operating element
e.g. <Save>
Menu - Submenu Specifies the menu path Settings - Time
1.3 Liability
In the case of damage and/or defects resulting from improper installation, assembly, or use of
the product, or from failure to observe the instructions for use and/or the safety instructions, any
liability on the part of BEMER Int. AG may be reduced or ruled out altogether and our warranty
obligations may cease to apply. In the aforementioned cases, no warranty claims will be accepted.
1.4 Copyright notice
All contents of these instructions for use, in particular texts, photographs, and graphics, are protected
by copyright. This form of legal protection also applies to databases and similar facilities. No part
of these instructions for use may be reproduced in any form outside the narrow framework of
copyright law without the written permission of BEMER Int. AG.
Anyone who infringes copyright (e.g., copies images or texts without permission) may be liable to
prosecution, receive a warning combined with a financial penalty, or be required to pay damages.
We reserve the right to enforce our rights in such cases.
RO SR PL SK CZ HU EN
98

1.5 Warranty
Warranty conditions
When you purchase BEMER products, you are given the opportunity to find out about our warranty
conditions. You can also view the current warranty conditions at any time in the relevant section
of our websites.
Your statutory rights in the event of defects are not restricted by our warranty terms; you can there-
fore assert them free of charge. Consequently, your existing statutory warranty rights in relation to
our products shall remain unaffected by our warranty commitment. The manufacturer’s warranty
terms therefore do not infringe your statutory rights, but rather strengthen your legal position.
1.6 Notification of incidents
If serious incidents occur while using this product, both the manufacturer (BEMER Int. AG) and
the competent authorities in the regions in which the user is based must be informed.
BEMER products may only be used for the purposes described in this chapter. Any use of the
product beyond the scope of the intended use is regarded as improper.
2.1 Intended use
2.1.1 Intended purpose
The B.Box Evo product, together with the PEMF applicators, the B.Light Clear Evo, and the B.Light
Restore Evo, is part of the “BEMER Therapy System Evo”.
The BEMER B.Box Evo is only used to generate the electrical signal that is used for the pulsed
electromagnetic field therapy (PEMF) and the low-level light therapy (LLLT), and to control the
individual programs.
In the context of pulsed electromagnetic field therapy (PEMF), the PEMF applicators are used in
conjunction with the B.Box Evo to stimulate blood flow in the small and very small blood vessels
(microcirculation), and to alleviate certain clinical conditions.
The B.Light Clear Evo emits light with wavelengths of 465 nm and 645 nm (±20 nm) and is used in
the context of low-level light therapy (LLLT) in conjunction with the B.Box Evo for skin treatment
on or near the skin surface.
The B.Light Restore Evo emits light with wavelengths of 645 nm and 860 nm (±20 nm) and is used
in the context of low-level light therapy (LLLT) in conjunction with the B.Box Evo for skin treatment
on or near the skin surface.
2.1.2 Medical applications
The B.Box Evo serves as the interface between the applicators and the users for LLLT and PEMF
applications.
2.1.3 Indications for use
The B.Box Evo has no indications for use per se.
The approved indications for use for PEMF and LLLT are defined by the applicators.
PEMF therapy is an adjuvant application and cannot replace medically prescribed therapy.
In the case of pre-existing conditions, the described user groups are limited to the following under-
lying conditions, their consequences and / or accompanying symptoms:
―Impaired wound healing
―Degenerative diseases of the musculoskeletal system
―Polyneuropathy due to diabetes mellitus or after cancer treatment
2. Safety
RO SR PL SK CZ HU EN
1110
―Chronic fatigue, e.g., associated with chronic stress or multiple sclerosis
―Acute and chronic pain
LLLT is an adjuvant application and cannot replace medically prescribed therapy. It supports
the treatment of skin diseases and is also intended as a supplement for cosmetic treatment.
Example applications for the B.Light Clear Evo:
―Treatment of mild to moderate acne vulgaris
―Improvement of the general appearance of the skin
―To help reduce inflammation (acne vulgaris)
Example applications for the B.Light Restore Evo:
―Cosmetic use: to help reduce the appearance of wrinkles and fine lines,
to improve skin texture
―To support wound healing
―To help reduce inflammation
―To support muscles and joints
2.1.4 Contraindications
The B.Box Evo has no contraindications per se.
Contraindications for PEMF and LLLT are defined by the applicators.
PEMF therapy is contraindicated for the following user groups:
―Individuals fitted with active medical implants (e.g., medication pumps, pacemakers)
―Recipients of organ transplants, allogeneic cell transplants, bone marrow or stem cell
transplants in combination with immunosuppressive therapy
(= intentional suppression of the immune system)
LLLT therapy (B.Light Clear Evo, B.Light Restore Evo) is contraindicated for the following applications:
―Do not apply directly to mucous membranes or in the vicinity of the eye
2.1.5 Potential side effects
The B.Box Evo has no potential side effects per se.
The potential side effects of PEMF und LLLT are defined by the applicators.
When PEMF therapy is applied via the corresponding PEMF applicators, the following short-term
side effects may occur in very rare cases:
―Change in pulse rate
―Change in blood pressure
When using LLL therapy by means of the associated light applicator modules (B.Light Clear Evo, B.Light
Restore Evo), the following localized and short-term skin reactions may occur in very rare cases:
―Redness of the skin (erythema)
―Itching
―Burning / stinging
―Dry skin
―Hyperpigmentation
2.1.6 Intended use
The intended use is defined by the applicators.
The PEMF applicators are used together with the B.Box Evo for regular systemic application and
additional targeted treatment. Depending on the applicator, different forms of application are possible.
Systemic (regular application / see chapter 6, basic plan):
B.Body Evo and B.Bed Evo are used for full-body therapy in the prone state.
Localized (optional):
B.Pad Evo, B.Sit Evo, and B.Spot Evo are additionally used for targeted treatment of individual
body regions.
The B.Grip Evo holder module (Class I) is required for use of the B.Spot Evo.
The LLLT application modules B.Light Clear Evo and B.Light Restore Evo are used in conjunction with
the B.Box Evo for targeted local application of polychromatic light on or near the skin surface.
The B.Grip Evo holder module (Class I) is required for use of the B.Light Clear Evo.
2.1.7 Intended users
The system is intended for use by end users aged 14 years and older, as well as by medically trained
personnel.
RO SR PL SK CZ HU EN
1312
2.1.8 Intended patient group
The device is intended for use on people aged 14 years or older in accordance with the respective
indications for use and contraindications.
Children under 14 years of age and individuals with impaired physical, sensory or mental capabilities
must be supervised and / or instructed by a suitable person who accepts responsibility for their safety.
2.1.9 Body regions
The body region to be treated is defined by the applicators.
The whole-body applicators (B.Body Evo and B.Bed Evo) are designed for systemic application of
the PEMF therapy. Due to the distribution of the coils, all regions of the body are reached by the
magnetic field.
The local applicators (B.Spot Evo, B.Pad Evo and B.Sit Evo) are optionally used for targeted application
of the magnetic field in specific areas of the body.
The LLLT applicators are optionally used for targeted application of LLLT on specific areas of the body.
2.1.10 Intended environment and field of application
The product is intended exclusively for the application of PEMF and LLLT in conjunction with the
applicators by laypersons in a home environment and by professional users in a clinical setting.
The PEMF applicators and LLT applicator modules, in combination with the B.Box Evo, are intended
for use by laypersons in a home environment and by medically trained personnel in a clinical or
outpatient setting.
2.2 Clinical benefits
The B.Box Evo itself has no clinical utility.
Users of PEMF therapy benefit from improved blood flow to the capillary network, in particular in
the smaller and smallest blood vessels, and thus from improved tissue supply, which is desirable
for various health conditions.
The application of LLLT using the B.Light Clear Evo leads to an improvement in the skin’s appearance,
especially in cases of mild to moderate acne vulgaris.
The application of LLLT using the B.Light Restore Evo leads to an improvement in the skin’s appearance.
2.3 General safety instructions
Interference with active implants due to electromagnetic forces (PEMF)
Active implants (e.g., pacemakers, insulin pumps, etc.) may be disrupted by electro-
magnetic forces.
zDo not use the BEMER Therapy System Evo (PEMF) under any circumstances if
you are a patient with an active implant.
Risk of strangulation due to loose cables
Loose cables and leads pose a risk of injury, e.g., caused by tripping or strangulation.
zLay the cables flat to ensure they do not create a tripping hazard.
zUse the supplied fastening aids to lay the cables.
Risk of burns due to damaged or worn out B.Box Evo Battery Stand
The failure of safety mechanisms may lead to spontaneous combustion or explosions.
zNever open or use lithium batteries or accumulators that are swollen, deformed,
outgassed, have “leaked”, or are covered with a “greasy film” or external deposits in
the area of the poles are damaged. Damaged batteries are associated with an
increased hazard risk.
zTherefore, dispose of these batteries and rechargeable batteries immediately,
preferably at an electronics store or recycling center – and in a manner that ensures
that the staff there can accept them.
zSpeak to the qualified personnel there and point out the damage.
Life-threatening hazards due to non-compliance with the safety instructions
Failure to follow the instructions for use correctly may result in operating errors and
life-threatening hazards.
zAlways read and familiarize yourself with the instructions for use included
in the scope of delivery.
zObserve the safety notes.
WARNING
RO SR PL SK CZ HU EN
1514
Risk of electric shock due to use of a damaged power cable or a power cable
not approved by the manufacturer
Contact with exposed electrical parts or power cables not approved by the manufacturer
may result in electric shock.
zDisconnect the device from the power supply.
zOnly use the power cables approved by the manufacturer.
Electric shock due to use of the device in a humid environment
Water and electricity are a dangerous combination that can cause an electric shock.
zDo not use this device in a humid environment (e.g., in the bathroom or near a
shower or swimming pool).
zDo not allow water to run into the unit.
Electric shock due to incorrect electrical voltage
Incorrect electrical voltage from the local power grid may cause electric shock and
permanently damage the device.
zCheck that the voltage specified on the device matches the local mains voltage
before connecting the device to avoid the risk of electric shock or permanent
damage to the device.
Risk of infection due to transmission of disease carriers
Shared use of the application modules can lead to the transmission of diseases.
zBetween each use, clean the applicators using the cleaning and disinfecting agent
recommended by the manufacturer.
WARNING
Risk of blinding due to optical radiation
If the B.Light Clear Evo or B.Light Restore Evo is used in the vicinity of the eye, there is a risk
of injury to the retina.
zAlways wear the supplied safety glasses when using the B.Light Evo application
modules.
Toxic skin reactions during application with ointments and medications
Use of the B.Light Clear Evo and / or B.Light Restore Evo light application module in
conjunction with light-intensive or light-reactive ointments or medications may result
in toxic skin reactions.
zDo not use light therapy in combination with ointments or medications.
CAUTION
Risk of burns / fire hazard due to overheated equipment
Overheated unattended equipment may result in an increased fire hazard and therefore
burns.
zTo avoid the risk of fire or burns, do not leave the unit unattended when it is
switched on.
zThe device must not be used by individuals with reduced physical, sensory or mental
faculties and / or who lack the necessary experience and knowledge, unless they
are supervised or given instructions on how to use the equipment to avoid the risk
of fire or burns.
zThe device is not intended for use by children. Ensure that children are supervised
and do not play with the device to avoid the risk of fire and burns.
Risk of infection due to application on injured skin
The use of contaminated application modules on injured skin can result in the transmission
of diseases.
zDo not apply the application modules to injured skin.
zBetween each use, clean and disinfect the applicators using the cleaning and
disinfecting agent recommended by the manufacturer.
WARNING
RO SR PL SK CZ HU EN
1716

Risk of burns due to high leakage currents
Simultaneous contact with two metallic parts can result in high leakage currents, which
can cause skin burns.
zDo not touch any metallic parts during use.
Electric shock due to water entering the device
Water ingress may cause a short circuit in the device and endanger the user.
zDisconnect live parts from the power supply before cleaning them.
zThe electrical contacts on the B.Box EvoRechargeable Battery (B.Box Evo Battery
Stand) must not come into contact with liquids.
Crushing injuries due to components with magnets
When the B.Grip Evo holder module is placed in close proximity to the B.Spot Evo, B.Light
Clear Evo, and B.Light Restore Evo application modules, skin pinching may occur due to the
resulting magnetic attraction. Skin bruising may also occur when attaching the B.Box Evo
to the B.Box Evo Stand or the B.Box Evo Battery Stand.
zFollow the instructions on the corresponding components and do not reach
between the holder module and the application modules or the B.Box Evo and the
B.Box Evo Stand or the B.Box Evo Battery Stand.
Increase in vital signs due to incorrect operation of the device
User errors may result in elevated vital signs, e.g., higher blood pressure, especially
among untrained users.
zWhen purchasing the BEMER Therapy System Evo, ensure that you are given an
introduction to the system by an official and certified BEMER partner.
zAlways read and familiarize yourself with the instructions for use included in the
scope of delivery.
zObserve the safety notes.
CAUTION
Increased surface temperature of the LLLT application modules
due to high ambient temperature
At ambient temperatures above 35°C, the surface of the light therapy application
modules can reach 44°C.
zIf the ambient temperature exceeds 35°C, allow the light therapy application
modules to cool for at least 10 minutes between treatments.
Performance degradation of portable RF communication devices
due to electromagnetic forces
The performance of the respective devices may be affected by electromagnetic forces.
zDo not use portable RF communication devices (including accessories such as
antenna cables and external antennas) within 12 inches (30cm) of any part of the
BEMER Therapy System Evo, including the cables specified by the manufacturer.
Using the device in an unsuitable environment
Use of this device in the immediate vicinity of other equipment and / or in a humid
environment can lead to malfunctions.
zDo not use this device when it is directly adjacent to or stacked on/under other
equipment.
zIf such use is unavoidable, monitor the device as well as the other equipment to
ensure normal operation.
zOnly use the BEMER Therapy System Evo in dry rooms.
NOTE
Allergic reactions due to material incompatibility
The materials used in the applicators may cause skin reactions due to intolerances.
zIf this occurs, discontinue use of the therapy system and consult your doctor.
CAUTION
RO SR PL SK CZ HU EN
1918
NOTE
There are no user-replaceable parts and no maintenance is required during
the service life of the system.
2.4 Medical notes
2.4.1 Medical notes on PEMF
In case of atypical reactions to the PEMF therapy, a doctor should be consulted.
For conditions requiring immunosuppression that are not related to transplantation, e.g.,
autoimmune diseases or dermatological diseases, there are no contraindications for use of
the PEMF therapy.
At the end of the first cycle, new users who regularly take blood thinning/anticoagulant agents
or antihypertensive medications are advised to consult their attending physician to check for
any change in their effectiveness.
If the following circumstances or complaints exist, the prior approval of the attending physician/
specialist must be obtained before application of the PEMF therapy:
―Unexplained fever
―Infectious diseases
―Severe cardiac rhythm disorder
―Severe psychosis
―Uncontrolled seizure disorders (e.g., epilepsy)
―Long-term use of β-recepter antagonists
―Use of high-dose corticoid agents
―Long-term use of anticoagulants (coumarin derivatives)
―Ongoing use of prescription medications
―Pregnancy
―Tumor diseases
2.4.2 Medical notes on LLLT
In case of atypical reactions to LLLT, a doctor should be consulted.
If the following circumstances or complaints exist, the prior approval of the attending physician/
specialist must be obtained before application of LLLT:
―Light-induced seizures (light sensitivity)
―Light-induced migraine headaches
―Ingestion or application of ointments (cosmetics), medications, or supplements known to
cause photosensitivity
―Ongoing use of prescription medications
―Allergic reaction caused by light
―Tumor diseases
―Cancerous lesions on the skin
―Skin lesions caused by bacteria, viruses or fungi
Electronic storage media can be corrupted or erased
The integral magnets in the B.Box Evo Stand and some of the cable connectors are very
strong. Electromagnetic fields can disrupt the functions of storage media (e.g., credit and
EC cards, data carriers) and can erase their contents.
zKeep storage media of this kind away from the magnets.
Property damage due to maintenance and repairs by unauthorized personnel
Repairs and maintenance activities performed by unauthorized and / or unqualified
individuals may result in material damage to the device.
zMaintenance and repair work may only be carried out by authorized personnel.
RO SR PL SK CZ HU EN
2120

2.5 Notes for professional users of medical products
Professional users must ensure that the respective employees are aware of and implement the
applicable occupational health and safety requirements. Furthermore, they must ensure that all
employees have read and understood the instructions for use.
Professional users must train their employees at regular intervals, inform them about
potential hazards, and provide them with the necessary protective equipment.
Individuals who are being trained, instructed, or are considered trainees in general may only work
on the BEMER Therapy System Evo under the constant supervision of an experienced person.
Work on electrical components may only be carried out by qualified personnel with appropriate
training and in compliance with all applicable provisions of the accident prevention regulations.
A safety check must be carried out by the operator at regular intervals.
2.6 Safety labels
2.7 Protective equipment
The Beauty Pack Evo includes the following protective equipment
―B.Light Evo Safety Glasses
The B.Light Evo Safety Glasses must be worn when using the B.Light Clear Evo
or the B.Light Restore Evo.
2.8 Environmental protection
BEMER Int. AG manufactures state-of-the-art therapy systems in terms of safety and environmental
protection. These therapy systems do not pose any danger to human health or the environment,
provided that they are operated properly.
Batteries contain toxic heavy metals. They are subject to hazardous waste treatment and
must be handed in at municipal collection points or disposed of by a specialist company.
Risk to human health and the environment due to toxic materials
Environmentally harmful materials, which the BEMER Therapy System Evo may
contain, pose a risk to human health and the environment.
zThe BEMER Therapy System Evo must not be disposed of with industrial or
household waste, either in whole or in part.
Symbol Definition City
All users must read the
instructions for use
This symbol is shown on
each product label
Patients with active implants
must not use the BEMER Therapy
System Evo (PEMF)
This symbol is shown
on the back of the B.Box Evo
Warning of high leakage currents This symbol is shown
on the inside of the B.Grip Evo
Warning of crushing injuries due to
components containing magnets
This symbol is shown
on the inside of the B.Grip Evo
CAUTION
RO SR PL SK CZ HU EN
2322

3. Technical data
3.1 Storage, transport, and operating conditions
3.2 Product-specific technical data
Temperature range (operating) +5 to 40°C
Humidity (operating) 15 to 90% (non-condensing)
Ambient air pressure (operating) 700 to 1060 hPa
Temperature range (storage, transport) -25 to +70°C
Humidity (storage, transport) 10 to 90% (non-condensing)
Ambient air pressure (storage, transport) 500 to 1060 hPa
Time until the operating temperature range is reached,
starting from the minimum transport temperature
~ 30 minutes
Time until the operating temperature range is reached,
starting from the maximum transport temperature
~ 30 minutes
Article number 424000
Product designation B.Box Evo
Product type Control unit
Dimensions (L x W x D) in mm 210 x 150 x 43
Weight (g) 926
Type Portable device
Surface material PC/ABS, aluminum, glass
IP degree of protection 22
Protection class (IEC 61140) SK II
Protection against electric shock 2MOPP Class II
EMC class (CISPR 11:2009) Class B
Input voltage 100 to 240 V AC / 50 to 60 Hz
Output voltage 15VDC / 2A
Operating voltage (V) 15
Max. power (watts) 30
Display dimensions (inches) 7"
Display resolution (px) 1024 x 600
Display brightness (cd/m²) 450
Display viewing angle (°) 80
Display contrast ratio 800:1
Location of type plate Rear of device
RO SR PL SK CZ HU EN
2524
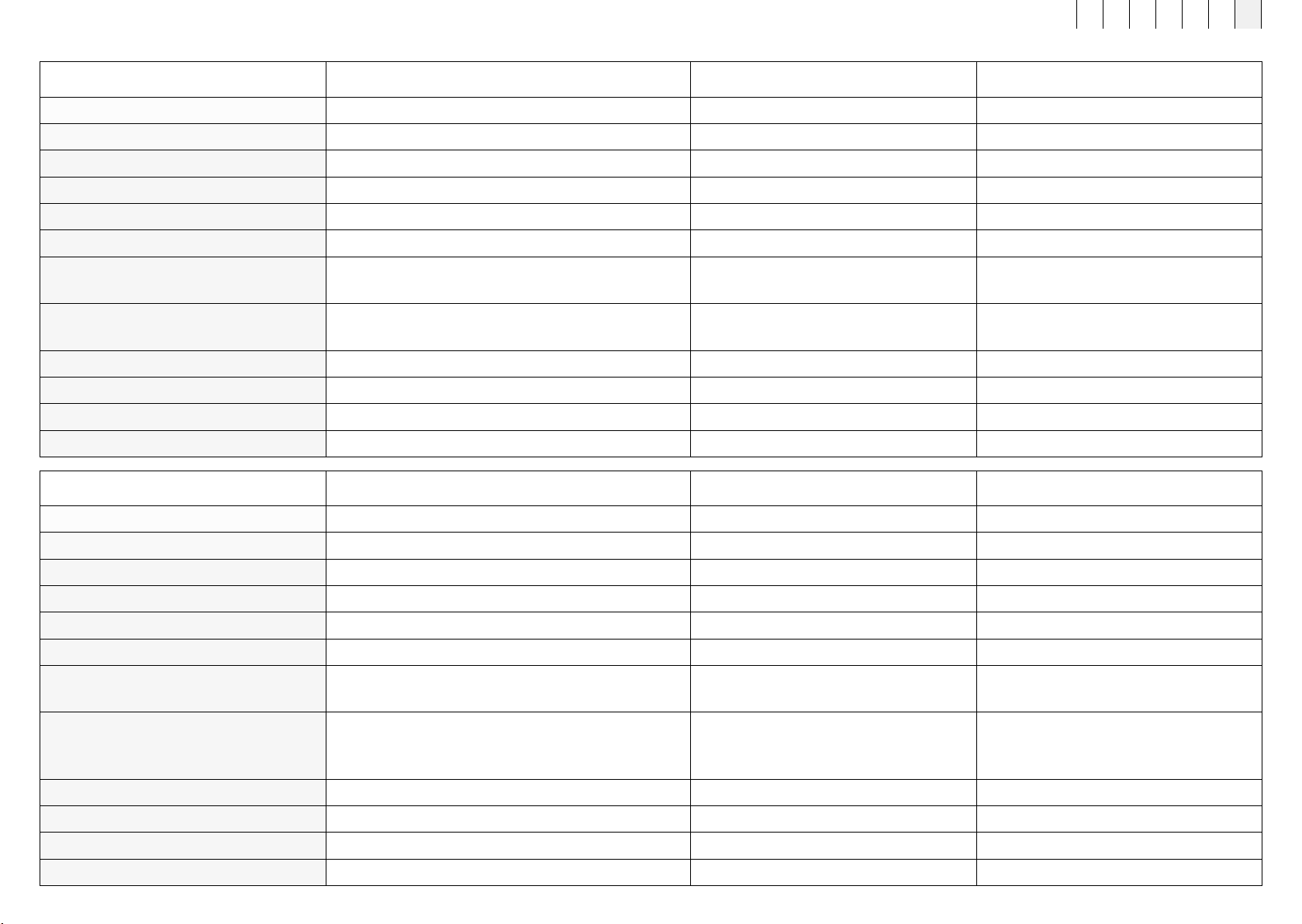
Designation B.Body Evo (full-body applicator) B.Bed Evo (full-body applicator) B.Pad Evo (local applicator)
Article number 434300 434400 434100
Dimensions L x W x D in cm 152 x 59 x 3 190 x 90 x 1 133 x 15 x 0.8
Weight in kg 1.9 1.97 0.33
Number of copper coils 16 16 4
Average flux density ≈ 35 μT (max. level) ≈ 35 μT (max. level) ≈ 100 μT (max. level)
Average flux density plus ≈ 50 μT (max. level) ≈ 50 μT (max. level) ≈ 150 μT (max. level)
Number of external connections 1 x magnetic connector with flexible cable
with PVC insulation
1 x magnetic connector with flexible cable
with PVC insulation
1 x magnetic connector with flexible cable
with PVC insulation
Composition of the
surface material in contact with the body
100% PES 100% PES 100% PES
Cable length 250 cm 250 cm 250 cm
Protection against moisture IP22 IP22 IP22
Device class Applied part, type BF Applied part, type BF Applied part, type BF
Location of type plate Rear of applicator Rear of applicator Rear of applicator
Designation B.Spot Evo (local applicator module) B.Sit Evo (local applicator) B.Grip Evo (holder module)
Article number 434000 434200 454000
Dimensions L x W x D in cm 12.12 x 12.12 x 2.46 44 x 36.7 x 5 12 x 12 x 4.8
Weight in kg 0.185 1.88 0.213
Number of copper coils 1 1 -
Average flux density ≈ 100 μT (max. level) ≈ 100 μT (max. level) -
Average flux density plus ≈ 150 μT (max. level) ≈ 150 μT (max. level) -
Number of external connections Rotation-free 5-pole tracks
1 x magnetic connector with flexible cable
with PVC insulation Rotation-free 5-pole tracks
Material composition (surface) 100% PC
Surface material in contact with the body
66% PES | 12% Rayon | 2% Spandex
20% TPU film PC/ABS
Cable length Cable on B.Grip Evo 250 cm 250 cm
Protection against moisture IP22 IP22 IP22
Device class Applied part, type BF Applied part, type BF -
Location of type plate Rear of applicator module Rear of applicator Inside of holder module
RO SR PL SK CZ HU EN
2726
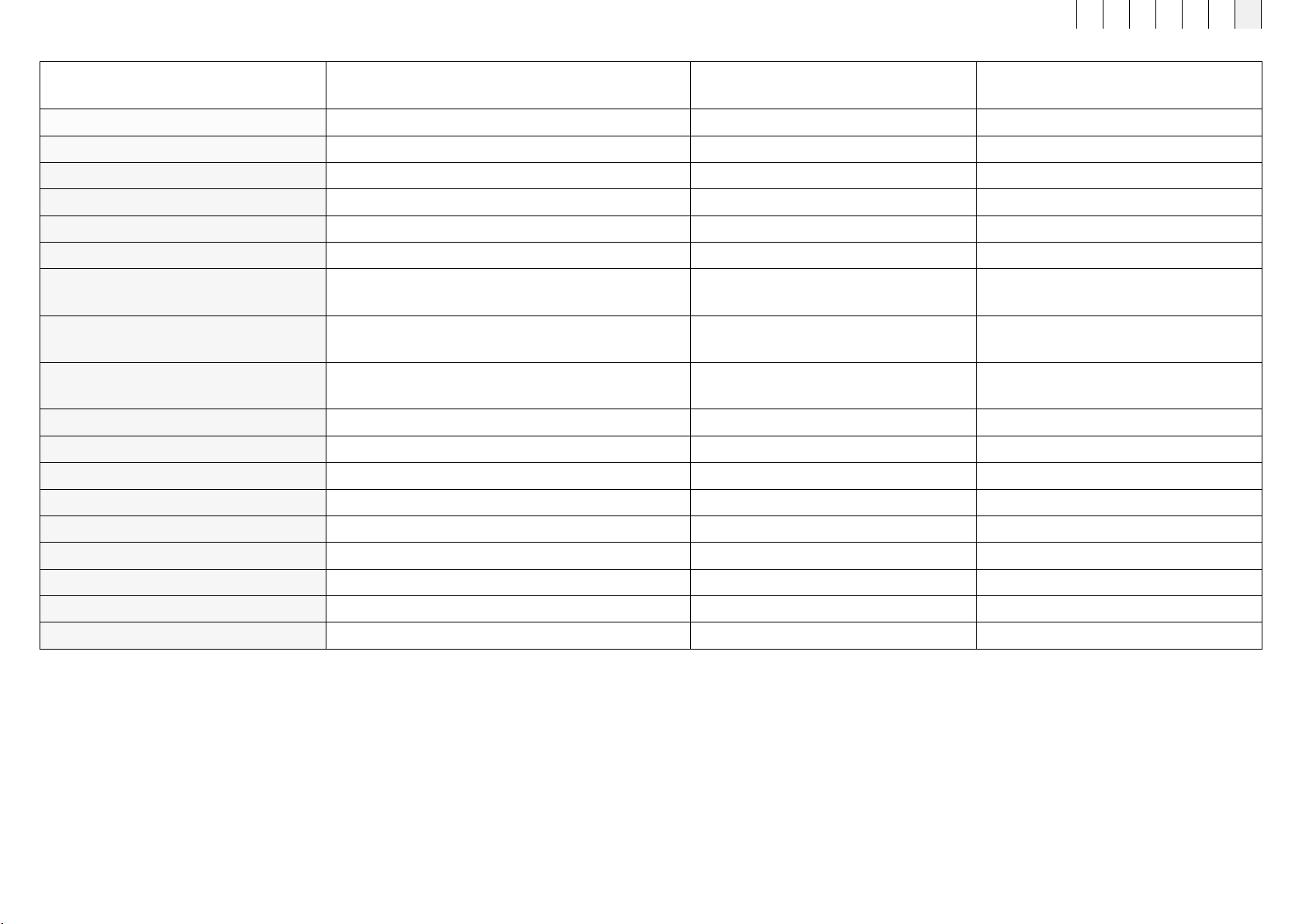
Designation
B.Light Clear Evo
(light applicator module)
B.Light Restore Evo
(light applicator module) B.Box Evo Battery Stand
Article number 434500 434600 454100
Dimensions L x W x D in cm 12.12 x 12.12 x 2.46 12.12 x 12.12 x 2.46 18.3 x 10.8 x 9.8
Weight in kg 0.12 0.12 0.498
Supply voltage - - 7.2 VDC
Wavelength range 465 nm and 645 nm (±20 nm) 645 nm and 860 nm (±20 nm) -
Number of LEDs 100 100 -
Maximum radiation intensity (mW/cm²)
at the skin surface
465 nm: ≈ 0.8
645 mm: ≈ 1.2
645 nm: ≈ 0.56
860 mm: ≈ 1.4 -
Treatment area
(J/cm2) on the skin during
a 480 sec. treatment
(J/cm2) on the skin during
a 480 sec. treatment -
Treatment dose (J/cm²) at the
skin surface per 480 sec. treatment ≈ 1 ≈ 1 -
Material composition (surface) 100% PC 100% PC 80 % Al, 20% PC/ABS
Protection against moisture IP22 IP22 IP22
Risk group 1 0 -
Device class Applied part, type BF Applied part, type BF -
Battery type - - Li-Ion
Battery capacity - - 48 Wh
Number of treatments - - ≈50 @ 8 minutes each
Number of external connections - - 5-pin connector
Location of type plate Rear of applicator module Rear of applicator module Underside of B.Box Evo Battery Stand
RO SR PL SK CZ HU EN
2928
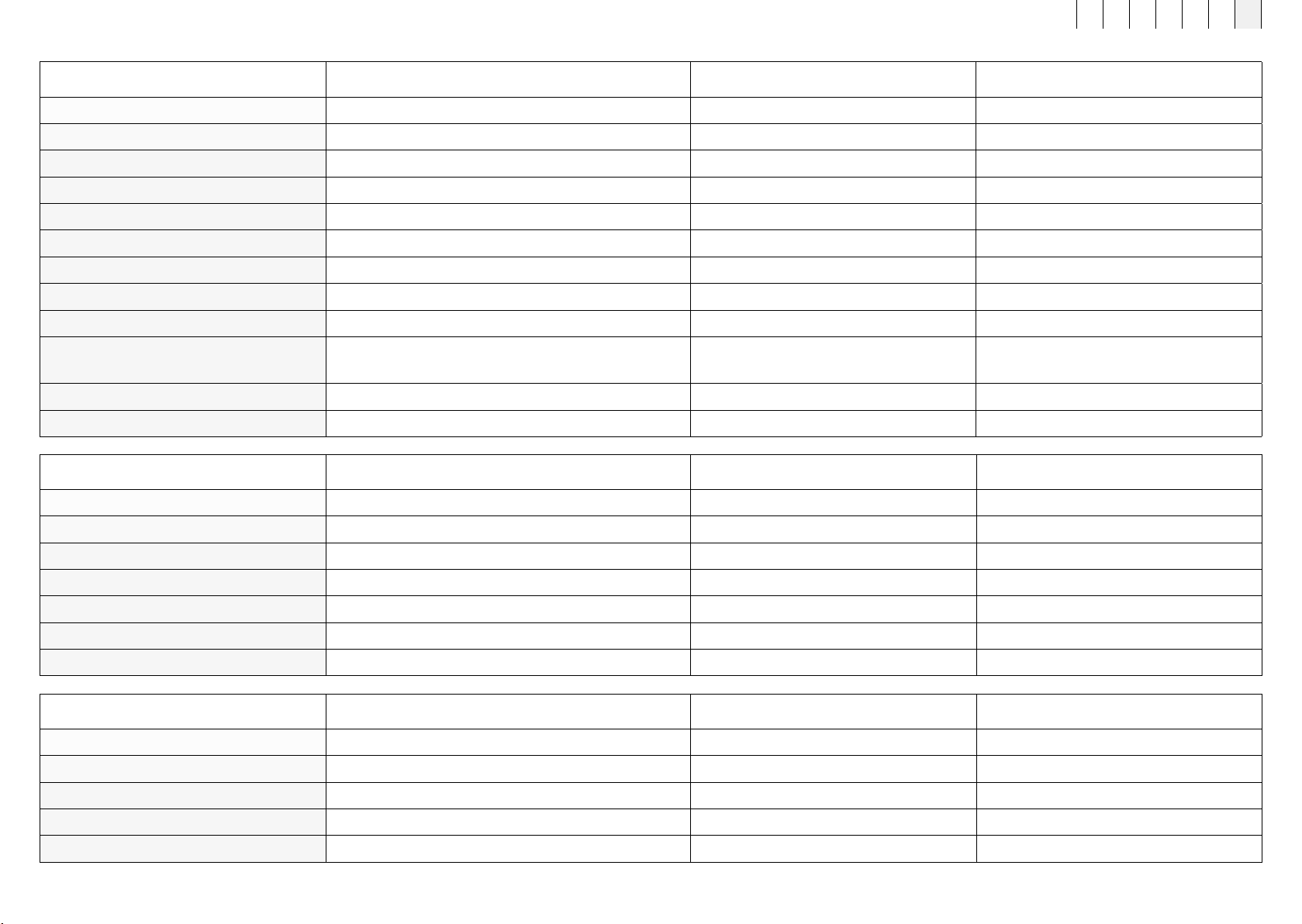
Designation B.BoxEvo Car Adapter B.Box EvoPower Supply B.Light Evo Safety Glasses
Article number 444100 444000 454900
Dimensions L x W x D in cm 10 x 4.5 x 2.25 10 x 4.5 x 2.25 14.1 x 6.2 x 4.5
Weight in kg 0.14 0.14 0.031
Primary voltage 10 to 32 VDC / 4A 100 to 240 VAC / 50–60Hz -
Number of external connections 1 - -
Cable length 360 cm 360 cm -
Power supply 15 V DC / 2A 15 V DC -
Protection class - SK II -
Protection against moisture IP21 IP21 IP22
Lenses - -
Shade 2, GA 166 CE
(anti-fog, scratch-resistant, 100% UV protection)
Material composition PC/ABS PC/ABS Polycarbonate (PC)
Location of type plate Underside of Car Adapter Underside of B.Box Evo Power Supply no type plate on safety glasses
Designation Extension cord for Evo applicators Velcro extension strap for B.Pad Evo B.Box Evo Stand
Article number 454500 454400 454800
Dimensions L x W x D in cm - 30.0 x 8.0 x 0.02 18 x 13 x 8
Weight in kg 0.118 0.031 0.48
Material composition Nylon / PC / ABS 85% PES, 15% Spandex Aluminium
Cable length, Ø in cm 250, 0.55 - -
Protection against moisture IP22 IP21 IP22
Location of type plate Product packaging Product packaging Underside of B.Box Evo Stand
Designation B.Grip Evo Fastening Strap B.Box Evo Wall Mount B.Bed Evo Fastening Strap
Article number 454200 454600 454700
Dimensions L x W x D in cm 80.2 x 9.2 x 1.2 17.9 x 12.3 x 1.2 Flexible length (elastic material)
Weight in kg 0.085 0.12 0.09
Material composition 50% PU, 40% PA, 10% SP PC/ABS, sheet steel Spandex
Location of type plate no type plate on Fastening Strap Underside of B.Box Evo Wall Mount Product packaging
RO SR PL SK CZ HU EN
3130

Designation B.BodyEvo foot protection
Article number 450500
Dimensions L x W x D in cm 67.5 x 60.0 x 0.02
Weight in kg 0.23
Material composition PES
Location of type plate Product packaging
3.3 EMC conformity
The BEMER therapy system Evo is intended for operation in an electromagnetic environment in
which immunity to RF disturbances is uncontrolled.
Electromagnetic Emission
Emission
Measurements Conformity
Electromagnetic Environment –
Guidlines
RF_emissions in
accordance with CISPR 11
Group 1 The BEMER therapy system uses RF energy
only for its internal function. As a result,
its RF emission is very low and it is unlikely
to disturb any adjacent electronic devices.
RF_emissions in
accordance with CISPR 11
Class B
The BEMER therapy system is intended for
use in all institutions including residential
areas and those that are directly connected
to a public grid that also supplies buildings
used for residential purposes.
Harmonics in accordance
with IEC 61000-3-2
Class A
Voltage fluctuations/flicker
in accordance with IEC
61000-3-3
Compliant
Recommended protective distances between portable and mobile RF telecommunication
devices and the BEMER therapy system
Customers or users can help to avoid electromagnetic disturbances by complying with the minimum
distance between portable and mobile RF telecommunication devices (transmitters) and the BEMER
therapy system, depending on the output of the communication device as indicated in the table on
the next page.
For transmitters where the maximum rated output is not listed in the table above, the distance can
be determined by using the equation that belongs to the relevant column, in which P is the maximum
rated output of the transmitter in watts (W) according to information from the transmitter manufacturer.
Rated output of
transmitter [W]
Protective distance,
depending on transmission frequency [m]
150 kHz to 80
MHz outside
ISM bands
150 kHz to 80
MHz inside
ISM bands
80 MHz to
800 MHz
800 MHz to
2,5 GHz
d= √P=1.17√P
3.5
3
d= √P=1.2√P
12
10 d= √P=1.2√P
12
10 d= √P=2.3√P
23
10
0,01 0,12 0,12 0,12 0,23
0,1 0,37 0,38 0,38 0,73
1 1,17 1,2 1,2 2,3
10 3,69 3,79 3,79 7,27
100 11,67 12 12 23
RO SR PL SK CZ HU EN
3332

Electromagnetic immunity
Emission measurements IEC-60601 test level Compliance level
Discharge of static electricity in accordance
with IEC 61000-4-2
± 8 kV contact discharge
± 15 kV air discharge
± 8 kV contact discharge
± 15 kV air discharge
Floors should be made of wood or concrete
or covered with ceramic tiles.
Electrical fast transient/burst immunity
in accordance with IEC 61000-4-4
± 2 kV for power cables
± 1 kV for input/output cables
± 2 kV for power cables
± 1 kV for input/output cables
The quality of the supply voltage should
correspond to that of a typical store or
clinic environment.
Impulse voltage/surges in accordance
with IEC 61000-4-5
± 1 kV voltage phase conductor –
phase conductor
± 2 kV voltage phase conductor – ground
± 1 kV voltage phase conductor –
phase conductor
± 2 kV voltage phase conductor – ground
Magnetic field at supply frequency (50/60 Hz)
in accordance with IEC 61000-4-8 3 A/m 3 A/m
Voltage dips, short-term interruptions
and fluctuations in the supply voltage
in accordance with IEC 61000-4-11
< 5 % UTfor 1/2 period (> 95 % dip)
< 40 % UTfor 10 periods (60 % dip)
< 70 % UTfor 25 periods (30 % dip)
< 5 % UT5 s (> 95 % dip)
< 5 % UTfor 1/2 period (> 95 % dip)
< 40 % UTfor 10 periods (60 % dip)
< 70 % UTfor 25 periods (30 % dip)
< 5 % UT5 s (> 95 % dip)
The quality of the supply voltage should
correspond to that of a typical store or clinic
environment. If the users of the device require
continuous operation, even in case of power
outages, it is recommended to feed power to
the device via an uninterruptible power
supply or a battery.
Radiated fields in close proximity in
accordance with IEC 61000-4-39
8 A/m at 30kHz
65 A/m at 134.2kHz 7.5 A/m at 13.56kHz
8 A/m at 30kHz
65 A/m at 134.2kHz 7.5 A/m at 13.56kHz
Avoid exposure to known sources of EMI
(electromagnetic interference) such as
diathermy, lithotripsy, electrocautery, RFID
(Radio Frequency Identification), and electro-
magnetic security systems such as anti-theft/
electronic article surveillance systems, metal
detectors. Note that the presence of RFID
devices may not be obvious. If such interfe-
rence is suspected, reposition the equipment
if possible, to maximize distances
Remark: UTis the AC mains voltage prior to applying the test level
RO SR PL SK CZ HU EN
3534

The applicators (B.Pad Evo, B.Body Evo, B.Bed Evo, and B.Sit Evo) and application modules (B.Spot
Evo, B.Light Restore Evo, B.Light Clear Evo) are operated via the controller (B.Box Evo). The applica-
tion modules additionally require the interchangeable adapter (B.Grip Evo).
Power is supplied either via the mains or the rechargeable B.Box Evo Battery Stand (optional
accessory). The medically approved B.Box Evo Car Adapter also allows use of the system via the
on-board power supply of a motor vehicle or boat. Only use the system when the vehicle is
stationary and ensure the B.Box Evo is appropriately secured.
4.1 System description
The BEMER Therapy System Evo is a medical product intended for daily use on humans, which
aims to stimulate microcirculation through the use of pulsed electromagnetic fields (PEMF). It is
also used for treatment of the skin through exposure to light of specific wavelengths (low-level
light therapy or LLLT).
The BEMER Therapy System Evo consists of various applicators (B.Body Evo, B.Bed Evo, B.Pad Evo
and B.Sit Evo) and applicator modules (B.Spot Evo, B.Light Clear Evo und B.Light Restore Evo). The
applicator modules are connected to the B.Box Evo controller together with the holder module
(B.Grip Evo). Without the B.Box Evo controller, neither the individual applicators nor the application
modules can be used.
4. BEMER Therapy System Evo
Immunity tests ICE-60601 test level Compliance level
Conducted RF disturbance in
accordance with IEC 61000-4-6
3 V
eff
150kHz to 80MHz inside the ISM/
amateur band
6 V
eff
150kHz to 80MHz inside the ISM/
amateur band
3V
eff
6V
eff
Radiated RF disturbance in
accordance with IEC 61000-4-3
10V/m
80 Mhz to 2.7GHz
10V/m
80 Mhz to 2.7GHz
Immunity to RF telecommunication equipment pursuant to limit values
of IEC 61000-4-3 tested and passed
Remark 1: The higher value applies for 80 MHz and 800 MHz.
Remark 2: These guidelines may not apply in all situations.The radiation of electromagnetic waves is
influenced by absorption and reflections from buildings, objects and people.
(a) Theoretically, the field strength of stationary transmitters such as the base stations of mobile
telephones and mobile terrestrial radio equipment, amateur radio stations, AM and FM radio and
television transmitters cannot be determined in advance. To determine the electromagnetic envi-
ronment with respect to the stationary transmitter, a study of the location should be considered.
If the measured field strength at the location at which the BEMER therapy system is used exceeds
the compliance level indicated above, the BEMER therapy system should be observed in order to
document its intended function. If atypical cable properties are observed, additional measures,
such as a change in alignment or a different location for the BEMER therapy system, may be necessary.
(b) The field strength should be lower than 3 V/m across the 150 kHz to 80 MHz frequency range.
Electromagnetic Environment – Guidelines
Recommended protective distance: d = 1.2 √P
d=1.2 √P for 80 MHz to 800 MHz
d=2,3 √P for 800 MHz to 2,5 GHz
With P as rated output of the transmitter in watts (W) pursuant to information from the transmitter
manufacturer and d as the recommended protective distance in meters (m). For all frequencies
pursuant to an examination on sitea, the field strength of stationary radio transmitters is lower
than the compliance leveld. Disturbances can occur in proximity to devices that are marked with
the following symbol:
RO SR PL SK CZ HU EN
3736

4.2 System overview
B.Box Evo
Applicators
B.Box Evo Battery Stand
B.BoxEvo Stand
B.Body Evo (PEMF) B.BodyEvo foot protection
Extension cord for
Evo applicators
B.BoxEvo Car Adapter
B.Box Evo Wall Mount
B.BedEvo Attachment Strap
Velcro extension strap for
B.PadEvo
B.Light Evo Safety Glasses
B.Pad Evo (PEMF)
B.Grip Evo
B.GripEvo Attachment Strap
B.Light Clear Evo (LLLT) B.Light Restore Evo (LLLT) B.Sit Evo (PEMF)
B.Bed Evo (PEMF)
B.Spot Evo (PEMF)
B.Box EvoPower Supply
Accessories
Controller with accessories
RO SR PL SK CZ HU EN
3938
Other manuals for Therapy System Evo
1
Table of contents
Languages:
Other Bemer Personal Care Product manuals





















